Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells
- PMID: 14709721
- PMCID: PMC3368615
- DOI: 10.1242/jcs.00893
Mechanism of recruiting Sec6/8 (exocyst) complex to the apical junctional complex during polarization of epithelial cells
Abstract
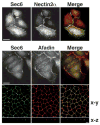
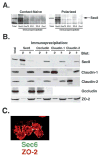
Sec6/8 (exocyst) complex regulates vesicle delivery and polarized membrane growth in a variety of cells, but mechanisms regulating Sec6/8 localization are unknown. In epithelial cells, Sec6/8 complex is recruited to cell-cell contacts with a mixture of junctional proteins, but then sorts out to the apex of the lateral membrane with components of tight junction and nectin complexes. Sec6/8 complex fractionates in a high molecular mass complex with tight junction proteins and a portion of E-cadherin, and co-immunoprecipitates with cell surface-labeled E-cadherin and nectin-2alpha. Recruitment of Sec6/8 complex to cell-cell contacts can be achieved in fibroblasts when E-cadherin and nectin-2alpha are co-expressed. These results support a model in which localized recruitment of Sec6/8 complex to the plasma membrane by specific cell-cell adhesion complexes defines a site for vesicle delivery and polarized membrane growth during development of epithelial cell polarity.
Figures






References
-
- Ando-Akatsuka Y, Yonemura S, Itoh M, Furuse M, Tsukita S. Differential behavior of E-cadherin and occludin in their colocalization with ZO-1 during the establishment of epithelial cell polarity. J Cell Physiol. 1999;179:115–125. - PubMed
-
- Aoki J, Koike S, Asou H, Ise I, Suwa H, Tanaka T, Miyasaka M, Nomoto A. Mouse homolog of poliovirus receptor-related gene 2 product, mPRR2, mediates homophilic cell aggregation. Exp Cell Res. 1997;235:374–384. - PubMed
-
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. New York: John Wiley and Sons; 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

