Roles of G-protein-coupled receptor dimerization
- PMID: 14710183
- PMCID: PMC1298963
- DOI: 10.1038/sj.embor.7400052
Roles of G-protein-coupled receptor dimerization
Abstract
The classical idea that G-protein-coupled receptors (GPCRs) function as monomeric entities has been unsettled by the emerging concept of GPCR dimerization. Recent findings have indicated not only that many GPCRs exist as homodimers and heterodimers, but also that their oligomeric assembly could have important functional roles. Several studies have shown that dimerization occurs early after biosynthesis, suggesting that it has a primary role in receptor maturation. G-protein coupling, downstream signalling and regulatory processes such as internalization have also been shown to be influenced by the dimeric nature of the receptors. In addition to raising fundamental questions about GPCR function, the concept of dimerization could be important in the development and screening of drugs that act through this receptor class. In particular, the changes in ligand-binding and signalling properties that accompany heterodimerization could give rise to an unexpected pharmacological diversity that would need to be considered.
Figures
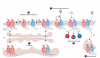

References
-
- AbdAlla S, Lother H, Quitterer U (2000) AT1–receptor heterodimers show enhanced G-protein activation and altered receptor sequestration. Nature 407: 94–98 - PubMed
-
- Angers S, Salahpour A, Bouvier M (2002) Dimerization: an emerging concept for G protein-coupled receptor ontogeny and function. Annu Rev Pharmacol Toxicol 42: 409–435 - PubMed
-
- Ayoub MA, Couturier C, Lucas-Meunier E, Angers S, Fossier P, Bouvier M, Jockers R (2002) Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J Biol Chem 277: 21522–21528 - PubMed
-
- Babcock GJ, Farzan M, Sodroski J (2003) Ligand-independent dimerization of CXCR4, a principal HIV-1 coreceptor. J Biol Chem 278: 3378–3385 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

