D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a
- PMID: 14710188
- PMCID: PMC1298959
- DOI: 10.1038/sj.embor.7400048
D4476, a cell-permeant inhibitor of CK1, suppresses the site-specific phosphorylation and nuclear exclusion of FOXO1a
Abstract
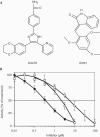
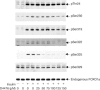
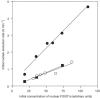
The protein kinase CK1 phosphorylates serine residues that are located close to another phosphoserine in the consensus pSer-Xaa-Xaa-Ser. This specificity generates regions in its target proteins containing two or more neighbouring phosphoserine residues, termed here multisite phosphorylation domains (MPDs). In this paper, we demonstrate that D4476 is a potent and rather selective inhibitor of CK1 in vitro and in cells. In H4IIE hepatoma cells, D4476 specifically inhibits the phosphorylation of endogenous forkhead box transcription factor O1a (FOXO1a) on Ser322 and Ser325 within its MPD, without affecting the phosphorylation of other sites. Our results indicate that these residues are targeted by CK1 in vivo and that the CK1-mediated phosphorylation of the MPD is required for accelerated nuclear exclusion of FOXO1a in response to IGF-1 and insulin. D4476 is much more potent and specific than IC261 or CKI-7, and is therefore the most useful CK1 inhibitor currently available for identifying physiological substrates of CK1.
Figures

Similar articles
-
CK1alpha plays a central role in mediating MDM2 control of p53 and E2F-1 protein stability.J Biol Chem. 2009 Nov 20;284(47):32384-94. doi: 10.1074/jbc.M109.052647. Epub 2009 Sep 15. J Biol Chem. 2009. PMID: 19759023 Free PMC article.
-
CK1 inhibitor affects in vitro maturation and developmental competence of bovine oocytes.Reprod Domest Anim. 2019 Aug;54(8):1104-1112. doi: 10.1111/rda.13483. Epub 2019 Jun 23. Reprod Domest Anim. 2019. PMID: 31155763
-
Two novel phosphorylation sites on FKHR that are critical for its nuclear exclusion.EMBO J. 2002 May 1;21(9):2263-71. doi: 10.1093/emboj/21.9.2263. EMBO J. 2002. PMID: 11980723 Free PMC article.
-
Achieving effective and selective CK1 inhibitors through structure modification.Future Med Chem. 2021 Mar;13(5):505-528. doi: 10.4155/fmc-2020-0215. Epub 2021 Jan 13. Future Med Chem. 2021. PMID: 33438471 Review.
-
Posttranslational regulation of Neurospora circadian clock by CK1a-dependent phosphorylation.Cold Spring Harb Symp Quant Biol. 2007;72:177-83. doi: 10.1101/sqb.2007.72.025. Cold Spring Harb Symp Quant Biol. 2007. PMID: 18419275 Review.
Cited by
-
Asymmetry of VANGL2 in migrating lymphocytes as a tool to monitor activity of the mammalian WNT/planar cell polarity pathway.Cell Commun Signal. 2015 Jan 28;13:2. doi: 10.1186/s12964-014-0079-1. Cell Commun Signal. 2015. PMID: 25627785 Free PMC article.
-
CK2 and protein kinases of the CK1 superfamily as targets for neurodegenerative disorders.Front Mol Biosci. 2022 Oct 6;9:916063. doi: 10.3389/fmolb.2022.916063. eCollection 2022. Front Mol Biosci. 2022. PMID: 36275622 Free PMC article. Review.
-
Casein Kinase 1 Regulates Cytorhabdovirus Replication and Transcription by Phosphorylating a Phosphoprotein Serine-Rich Motif.Plant Cell. 2020 Sep;32(9):2878-2897. doi: 10.1105/tpc.20.00369. Epub 2020 Jul 8. Plant Cell. 2020. PMID: 32641349 Free PMC article.
-
WNT signaling inducing activity in ascites predicts poor outcome in ovarian cancer.Theranostics. 2020 Jan 1;10(2):537-552. doi: 10.7150/thno.37423. eCollection 2020. Theranostics. 2020. PMID: 31903136 Free PMC article.
-
Breast cancer-specific mutations in CK1epsilon inhibit Wnt/beta-catenin and activate the Wnt/Rac1/JNK and NFAT pathways to decrease cell adhesion and promote cell migration.Breast Cancer Res. 2010;12(3):R30. doi: 10.1186/bcr2581. Epub 2010 May 27. Breast Cancer Res. 2010. PMID: 20507565 Free PMC article.
References
-
- Beals CR, Sheridan CM, Turck CW, Gardner P, Crabtree GR (1997) Nuclear export of NF-ATc enhanced by glycogen synthase kinase-3. Science 275: 1930–1933 - PubMed
-
- Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME (1999) Akt promotes cell survival by phosphorylating and inhibiting a forkhead transcription factor. Cell 96: 857–868 - PubMed
-
- Callahan JF et al. (2002) Identification of novel inhibitors of the transforming growth factor-1 (TGF-1) type 1 receptor (ALK5). J Med Chem 45: 999–1001 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

