First-line endocrine treatment of breast cancer: aromatase inhibitor or antioestrogen?
- PMID: 14710200
- PMCID: PMC2395329
- DOI: 10.1038/sj.bjc.6601508
First-line endocrine treatment of breast cancer: aromatase inhibitor or antioestrogen?
Abstract
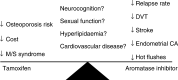
Until recently, endocrine therapy for breast cancer was relatively simple. If the tumour expressed hormone receptors, regardless of stage and age, tamoxifen was indicated. While this largely remains the case for premenopausal women, clinical trials in postmenopausal women have broadened our choice to include one of three selective aromatase inhibitors (AIs), the nonsteroidal agents anastrozole or letrozole and the steroidal agent exemestane. Comparative data concerning the efficacy, toxicity, tolerability and cost of AI vs tamoxifen continues to evolve with over 40 000 women slated to be involved in clinical trials. Currently, tamoxifen remains an appropriate choice for adjuvant treatment, and will remain so unless a clear survival advantage emerges for adjuvant AI therapy. However, anastrozole is widely seen as a useful alternative, with particular merit for patients prone to the development of serious tamoxifen side effects. For endocrine therapy naïve advanced disease, several trials have provided evidence that a nonsteroidal AI has replaced tamoxifen as optimal treatment. In the neoadjuvant setting, letrozole was also more effective than tamoxifen, both in terms of response rates and the incidence of breast-conserving surgery, and so AI therefore also dominates this evolving indication. The ongoing adjuvant clinical trials ask all the relevant questions regarding tamoxifen and AI in combination, sequence and duration, except for 5 years of an AI vs a longer period. For both the advanced and early-stage disease, resistance remains the key obstacle to overcome, and trials that combine endocrine agents with signal transduction inhibitors such as HER1 and HER2 kinase inhibitors, farnesyl transferase inhibitors, mTOR inhibitors as well as COX2 inhibitors are being developed in a concerted attempt to address this problem.
Figures
References
-
- ATAC (2002) Anastrozole alone or in combination with tamoxifen versus tamoxifen alone for adjuvant treatment of postmenopausal women with early breast cancer: first results of the ATAC randomised trial. Lancet 359: 2131–2139 - PubMed
-
- Bonneterre J, Buzdar A, Nabholtz JM, Robertson JF, Thurlimann B, von Euler M, Sahmoud T, Webster A, Steinberg M (2001) Anastrozole is superior to tamoxifen as first-line therapy in hormone receptor positive advanced breast carcinoma. Cancer 92: 2247–2258 - PubMed
-
- Bonneterre J, Thurlimann B, Robertson JF, Krzakowski M, Mauriac L, Koralewski P, Vergote I, Webster A, Steinberg M, von Euler M (2000) Anastrozole versus tamoxifen as first-line therapy for advanced breast cancer in 668 postmenopausal women: results of the Tamoxifen or Arimidex Randomized Group Efficacy and Tolerability study. J Clin Oncol 18: 3748–3757 - PubMed
-
- Brodie A, Jelovac D, Long BJ (2003) Predictions from a preclinical model: studies of aromatase inhibitors and antiestrogens. Clin Cancer Res 9: 455S–459S - PubMed
-
- Buzdar A (2002) The ATAC (‘Arimidex’, Tamoxifen, Alone or in Combination) trial in postmenopausal women with early breast cancer – updated efficacy results based on a median follow-up of 47 months (abstract 13). San Antonio Breast Cancer Symposium, San Antonio, Texas, USA
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous