Randomized Phase II trial assessing estramustine and vinblastine combination chemotherapy vs estramustine alone in patients with progressive hormone-escaped metastatic prostate cancer
- PMID: 14710214
- PMCID: PMC2395315
- DOI: 10.1038/sj.bjc.6601468
Randomized Phase II trial assessing estramustine and vinblastine combination chemotherapy vs estramustine alone in patients with progressive hormone-escaped metastatic prostate cancer
Abstract
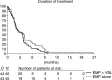
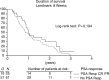
Based on the results of combined data from three North American Phase II studies, a randomised Phase II study in the same patient population was performed, using combination chemotherapy with estramustine phosphate (EMP) and vinblastine (VBL) in hormone refractory prostate cancer patients. In all, 92 patients were randomised into a Phase II study of oral EMP (10 mg kg day continuously) or oral EMP in combination with intravenous VBL (4 mg m(2) week for 6 weeks, followed by 2 weeks rest). The end points were toxicity and PSA response in both groups, with the option to continue the trial as a Phase III study with time to progression and survival as end points, if sufficient responses were observed. Toxicity was unexpectedly high in both treatment arms and led to treatment withdrawal or refusal in 49% of all patients, predominantly already during the first treatment cycle. The mean treatment duration was 10 and 14 weeks, median time to PSA progression was 27.2 and 30.8 weeks, median survival time was 44 and 50.9 weeks, and PSA response rate was only 24.6 and 28.9% in the EMP/VBL and EMP arms, respectively. There was no correlation between PSA response and survival. While the PSA response in the patients tested was less than half that recorded in the North American studies, the toxicity of EMP monotherapy or in combination with VBL was much higher than expected. Further research on more effective and less toxic treatment strategies for hormone refractory prostate cancer is mandatory.
Figures
References
-
- Amato RJ, Ellerhorst J, Bui C, Logothetis CJ (1995) Estramustine and vinblastine for patients with progressive androgen-independent adenocarcinoma of the prostate. Urol Oncol 1: 168–172 - PubMed
-
- Bauer KS, Figg WD, Hamilton JM, Jones EC, Premkumar A, Steinberg SM, Dyer V, Linehan WM, Pluda JM, Reed E (1999) A pharmacokinetically guided phase II study of carboxyamido-triazole (CAI) in androgen independent prostate cancer. Clin Cancer Res 5: 2324–2329 - PubMed
-
- Benson RC, Hartley-Asp B (1990) Mechanisms of action and clinical uses of estramustine. Cancer Invest 8: 375–380 - PubMed
-
- Bubley GJ, Carducci M, Dahut W, Dawson N, Daliani D, Eisenberger M, Figg WD, Freidlin B, Halabi S, Hudes G, Hussain M, Kaplan R, Myers C, Oh W, Petrylak DP, Reed E, Roth B, Sartor O, Scher H, Simons J, Sinibaldi V, Small EJ, Smith MR, Trump DL, Wilding G (1999) Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J Clin Oncol 17: 3461–3467 - PubMed
-
- Crawford ED, Eisenberger MA, McLeod MA, Spaulding JT, Benson R, Dorr FA, Blumenstein BA, Davis MA, Goodman PJ (1989) A controlled trial of leuprolide with and without flutamide in prostatic cancer. New Engl J Med 321: 419–924 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous