Encapsulation into sterically stabilised liposomes enhances the immunogenicity of melanoma-associated Melan-A/MART-1 epitopes
- PMID: 14710238
- PMCID: PMC2395333
- DOI: 10.1038/sj.bjc.6601473
Encapsulation into sterically stabilised liposomes enhances the immunogenicity of melanoma-associated Melan-A/MART-1 epitopes
Abstract
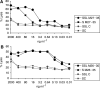
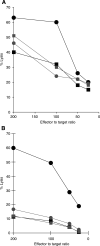
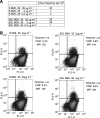
Tumour-associated antigens (TAA)-specific vaccination requires highly immunogenic reagents capable of inducing cytotoxic T cells (CTL). Soluble peptides are currently used in clinical applications despite an acknowledged poor immunogenicity. Encapsulation into liposomes has been suggested to improve the immunogenicity of discrete antigen formulations. We comparatively evaluated the capacity of HLA-A2.1 restricted Melan-A/MART-1 epitopes in soluble form (S) or following inclusion into sterically stabilised liposomes (SSL) to be recognised by specific CTL, to stimulate their proliferation and to induce them in healthy donors' peripheral blood mononuclear cells (PBMC), as well as in melanoma-derived tumour-infiltrating lymphocytes (TIL). HLA-A2.1(+), Melan-A/MART-1-NA-8 melanoma cells served as targets of specific CTL in 51Cr release assays upon pulsing by untreated or human plasma-treated soluble or SSL-encapsulated Melan-A/MART-1 27-35 (M27-35) or 26-35 (M26-35) epitopes. These reagents were also used to stimulate CTL proliferation, measured as 3H-thymidine incorporation, in the presence of immature dendritic cells (iDC), as antigen-presenting cells (APC). Induction of specific CTL upon stimulation with soluble or SSL-encapsulated peptides was attempted in healthy donors' PBMC or melanoma-derived TIL, and monitored by 51Cr release assays and tetramer staining. Na-8 cells pulsing with SSL M27-35 resulted in a five-fold more effective killing by specific CTL as compared with equal amounts of S M27-35. Encapsulation into SSL also provided a partial (50%) protection of M27-35 from plasma hydrolysis. No specific advantages regarding M26-35 were detectable in these assays. However, at low epitope concentrations (</=100 ng ml(-1)), SSL M26-35 was significantly more effective in inducing CTL proliferation than S M26-35, in the presence of iDC, as APC. Preincubation with iDC for 6 h virtually abolished the capacity of S M26-35 to stimulate specific CTL proliferation, but only partially affected that of SSL M26-35. Most importantly, SSL M26-35 was able to enhance the induction of specific CTL in healthy donors PBMC and in melanoma-derived TIL as compared to S M26-35. Taken together, our data indicate that encapsulation of TAA epitopes into SSL results in effective immunogenic formulations suitable for clinical use in active specific tumour immunotherapy.
Figures


References
-
- Agrawal B, Krantz MJ, Reddish MA, Longenecker BM (1998) Rapid induction of primary CD4+ and CD8+ T cell responses against cancer-associated MUC-1 peptide epitopes. Int Immunol 157: 2089–2095 - PubMed
-
- Albo F, Cavazza A, Giardina B, Lippa S, Marini M, Roda LG, Spagnoli G (2003) Degradation of the immunogenic peptide gp100 280–288 by the monocyte-like U937 cell line. Peptides 24: 371–378 - PubMed
-
- Allen TM (1994) Long-circulating (sterically stabilized) liposomes for targeted drug delivery. Trends Pharmacol Sci 15: 215–220 - PubMed
-
- Alving CR, Koulchin V, Glenn GM, Rao M (1995) Liposomes as carriers of peptide antigens: induction of antibodies and cytotoxic T lymphocytes to conjugated and unconjugated peptides. Immunol Rev 145: 5–31 - PubMed
-
- Amoscato AA, Prenovitz DA, Lotze MT (1998) Rapid extracellular degradation of synthetic class I peptides by human dendritic cells. J Immunol 161: 4023–4032 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

