Syndecans in tumor cell adhesion and signaling
- PMID: 14711376
- PMCID: PMC320497
- DOI: 10.1186/1477-7827-2-3
Syndecans in tumor cell adhesion and signaling
Abstract
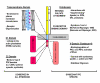
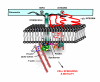
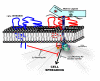
Anchorage of cells to "heparin"--binding domains that are prevalent in extracellular matrix (ECM) components is thought to occur primarily through the syndecans, a four-member family of transmembrane heparan sulfate proteoglycans that communicate environmental cues from the ECM to the cytoskeleton and the signaling apparatus of the cell. Known activities of the syndecans trace to their highly conserved cytoplasmic domains and to their heparan sulfate chains, which can serve to regulate the signaling of growth factors and morphogens. However, several emerging studies point to critical roles for the syndecans' extracellular protein domains in tumor cell behavior to include cell adhesion and invasion. Although the mechanisms of these activities remain largely unknown, one possibility involves "co-receptor" interactions with integrins that may regulate integrin function and the cell adhesion-signaling phenotype. Thus, alterations in syndecan expression, leading to either overexpression or loss of expression, both of which take place in tumor cells, may have dramatic effects on tumor cell invasion.
Figures
References
-
- Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–1164. - PubMed
-
- Brassard DL, Maxwell E, Malkowski M, Nagabhushan TL, Kumar CC, Armstrong L. Integrin alpha(v)beta(3)-mediated activation of apoptosis. Exp Cell Res. 1999;251:33–45. - PubMed
-
- Kuzuya M, Satake S, Ramos MA, Kanda S, Koike T, Yoshino K, Ikeda S, Iguchi A. Induction of apoptotic cell death in vascular endothelial cells cultured in three-dimensional collagen lattice. Exp Cell Res. 1999;248:498–508. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

