Differential stress-induced regulation of two quinone reductases in the brown rot basidiomycete Gloeophyllum trabeum
- PMID: 14711659
- PMCID: PMC321286
- DOI: 10.1128/AEM.70.1.324-331.2004
Differential stress-induced regulation of two quinone reductases in the brown rot basidiomycete Gloeophyllum trabeum
Abstract
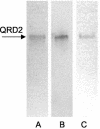
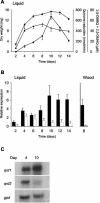
Quinone reductases (QRDs) have two important functions in the basidiomycete Gloeophyllum trabeum, which causes brown rot of wood. First, a QRD is required to generate biodegradative hydroxyl radicals via redox cycling between two G. trabeum extracellular metabolites, 2,5-dimethoxyhydroquinone (2,5-DMHQ) and 2,5-dimethoxy-1,4-benzoquinone (2,5-DMBQ). Second, because 2,5-DMBQ is cytotoxic and 2,5-DMHQ is not, a QRD is needed to maintain the intracellular pool of these metabolites in the reduced form. Given their importance in G. trabeum metabolism, QRDs could prove useful targets for new wood preservatives. We have identified two G. trabeum genes, each existing in two closely related, perhaps allelic variants, that encode QRDs in the flavodoxin family. Past work with QRD1 and heterologous expression of QRD2 in this study confirmed that both genes encode NADH-dependent, flavin-containing QRDs. Real-time reverse transcription PCR analyses of liquid- and wood-grown cultures showed that qrd1 expression was maximal during secondary metabolism, coincided with the production of 2,5-DMBQ, and was moderately up-regulated by chemical stressors such as quinones. By contrast, qrd2 expression was maximal during fungal growth when 2,5-DMBQ levels were low, yet was markedly up-regulated by chemical stress or heat shock. The total QRD activity in lysates of G. trabeum mycelium was significantly enhanced by induction beforehand with a cytotoxic quinone. The promoter of qrd2 contains likely antioxidant, xenobiotic, and heat shock elements, absent in qrd1, that probably explain the greater response of qrd2 transcription to stress. We conclude from these results that QRD1 is the enzyme G. trabeum routinely uses to detoxify quinones during incipient wood decay and that it could also drive the biodegradative quinone redox cycle. However, QRD2 assumes a more important role when the mycelium is stressed.
Figures


References
-
- Bolton, J. L., M. A. Trush, T. M. Penning, G. Dryhurst, and T. J. Monks. 2000. Role of quinones in toxicology. Chem. Res. Toxicol. 13:135-159. - PubMed
-
- Bustin, S. A. 2000. Absolute quantification of mRNA using real-time reverse transcription polymerase chain reaction assays. J. Mol. Endocrinol. 25:169-193. - PubMed
-
- Butler, J. E., and J. T. Kadonaga. 2002. The RNA polymerase II core promoter: a key component in the regulation of gene expression. Genes Dev. 16:2583-2592. - PubMed
-
- Cohen, R., K. A. Jensen, Jr., C. J. Houtman, and K. E. Hammel. 2002. Significant levels of extracellular reactive oxygen species produced by brown rot basidiomycetes on cellulose. FEBS Lett. 531:483-488. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

