Frequency, size, and localization of bacterial aggregates on bean leaf surfaces
- PMID: 14711662
- PMCID: PMC321242
- DOI: 10.1128/AEM.70.1.346-355.2004
Frequency, size, and localization of bacterial aggregates on bean leaf surfaces
Abstract
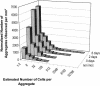
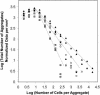
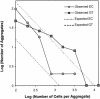
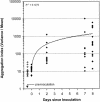
Using epifluorescence microscopy and image analysis, we have quantitatively described the frequency, size, and spatial distribution of bacterial aggregates on leaf surfaces of greenhouse-grown bean plants inoculated with the plant-pathogenic bacterium Pseudomonas syringae pv. syringae strain B728a. Bacterial cells were not randomly distributed on the leaf surface but occurred in a wide range of cluster sizes, ranging from single cells to over 10(4) cells per aggregate. The average cluster size increased through time, and aggregates were more numerous and larger when plants were maintained under conditions of high relative humidity levels than under dry conditions. The large majority of aggregates observed were small (less than 100 cells), and aggregate sizes exhibited a strong right-hand-skewed frequency distribution. While large aggregates are not frequent on a given leaf, they often accounted for the majority of cells present. We observed that up to 50% of cells present on a leaf were located in aggregates containing 10(3) cells or more. Aggregates were associated with several different anatomical features of the leaf surface but not with stomates. Aggregates were preferentially associated with glandular trichomes and veins. The biological and ecological significance of aggregate formation by epiphytic bacteria is discussed.
Figures



References
-
- Andersen, G. L., O. Menkissoglou, and S. E. Lindow. 1991. Occurrence and properties of copper-tolerant strains of Pseudomonas syringae isolated from fruit trees in California [USA]. Phytopathology 81:648-656.
-
- Ascensao, L., and M. S. Pais. 1998. The leaf capitate trichomes of Leonotis leonurus: histochemistry, ultrastructure and secretion. Ann. Bot. 81:263-271.
-
- Bailey, M. J., A. K. Lilley, and J. P. Diaper. 1996. Gene transfer between microorganisms in the phyllosphere, p 103-123. In C. E. Morris, P. C. Nicot, and C. Nguyen-The (ed.), Aerial plant surface microbiology. Plenum Press, New York, N.Y.
-
- Barber, C. E., J. L. Tang, J. X. Feng, M. Q. Pan, T. J. G. Wilson, H. Slater, J. M. Dow, P. Williams, and M. J. Daniels. 1997. A novel regulatory system required for pathogenicity of Xanthomonas campestris is mediated by a small diffusible signal molecule. Mol. Microbiol. 24:555-566. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

