Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation
- PMID: 14711988
- PMCID: PMC321769
- DOI: 10.1073/pnas.0304375101
Mislocalization to the nuclear envelope: an effect of the dystonia-causing torsinA mutation
Abstract
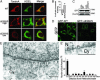
Primary dystonia is a disease characterized by involuntary twisting movements caused by CNS dysfunction without underlying histopathology. DYT1 dystonia is a form of primary dystonia caused by an in-frame GAG deletion (DeltaE302/3) in the TOR1A gene that encodes the endoplasmic reticulum luminal protein torsinA. We show that torsinA is also present in the nuclear envelope (NE), where it appears to interact with substrate, and that the DeltaE302/3 mutation causes a striking redistribution of torsinA from the endoplasmic reticulum to the NE. In addition, DeltaE302/3-torsinA recruits WT torsinA to the NE, potentially providing insight into an understanding of the dominant inheritance of the disease. DYT1 dystonia appears to be a previously uncharacterized NE disease and the first, to our knowledge, to selectively affect CNS function.
Figures





Comment in
-
TorsinA and torsion dystonia: Unraveling the architecture of the nuclear envelope.Proc Natl Acad Sci U S A. 2004 Jun 15;101(24):8839-40. doi: 10.1073/pnas.0402441101. Epub 2004 Jun 8. Proc Natl Acad Sci U S A. 2004. PMID: 15187229 Free PMC article. Review. No abstract available.
References
-
- Fahn, S., Marsden, C. D. & Calne, D. B. (1987) in Movement Disorders 2, eds. Marsden, C. D. & Fahn, S. (Butterworths, London), pp. 332–58.
-
- Berardelli, A., Rothwell, J. C., Hallett, M., Thompson, P. D., Manfredi, M. & Marsden, C. D. (1998) Brain 121, 1195–1212. - PubMed
-
- Ozelius, L. J., Hewett, J. W., Page, C. E., Bressman, S. B., Kramer, P. L., Shalish, C., de Leon, D., Brin, M. F., Raymond, D., Corey, D. P., et al. (1997) Nat. Genet. 17, 40–48. - PubMed
-
- Neuwald, A. F., Aravind, L., Spouge, J. L. & Koonin, E. V. (1999) Genome Res. 9, 27–43. - PubMed
-
- Ogura, T. & Wilkinson, A. J. (2001) Genes Cells 6, 575–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

