Hydrogen storage in molecular compounds
- PMID: 14711993
- PMCID: PMC321744
- DOI: 10.1073/pnas.0307449100
Hydrogen storage in molecular compounds
Abstract
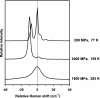
At low temperature (T) and high pressure (P), gas molecules can be held in ice cages to form crystalline molecular compounds that may have application for energy storage. We synthesized a hydrogen clathrate hydrate, H(2)(H(2)O)(2), that holds 50 g/liter hydrogen by volume or 5.3 wt %. The clathrate, synthesized at 200-300 MPa and 240-249 K, can be preserved to ambient P at 77 K. The stored hydrogen is released when the clathrate is warmed to 140 K at ambient P. Low T also stabilizes other molecular compounds containing large amounts of molecular hydrogen, although not to ambient P, e.g., the stability field for H(2)(H(2)O) filled ice (11.2 wt % molecular hydrogen) is extended from 2,300 MPa at 300 K to 600 MPa at 190 K, and that for (H(2))(4)CH(4) (33.4 wt % molecular hydrogen) is extended from 5,000 MPa at 300 K to 200 MPa at 77 K. These unique characteristics show the potential of developing low-T molecular crystalline compounds as a new means for hydrogen storage.
Figures


References
-
- Dresselhaus, M. S. & Thomas, I. L. (2001) Nature 414, 332–337. - PubMed
-
- Schlapbach, L. & Züttel, A. (2001) Nature 414, 353–358. - PubMed
-
- Loveday, J. S., Nelmes, R. J., Guthrie, M., Belmonte, S. A., Allan, D. R., Klug, D. D., Tse, J. S. & Handa, Y. P. (2001) Nature 410, 661–663. - PubMed
-
- Hirai, H., Uchihara, Y., Fujihisa, H., Sakashita, M., Katoh, E., Aoki, K., Nagashima, K., Yamamoto, Y. & Yagi, T. (2001) J. Chem. Phys. 115, 7066–7070.
LinkOut - more resources
Full Text Sources
Other Literature Sources

