Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis
- PMID: 14712306
- PMCID: PMC2825886
- DOI: 10.1038/sj.gt.3302143
Altered expression of antiviral cytokine mRNAs associated with cyclophosphamide's enhancement of viral oncolysis
Abstract
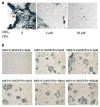
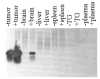
Oncolytic viruses (OVs) are being used as anticancer agents in preclinical and clinical trials. Propagation of OVs inside infected tumors is critical to their efficacy and is mediated by the productive generation of progeny OVs within infected tumor cells. In turn, this progeny can spread the infection to other tumor cells in successive rounds of oncolysis. Previously, we had found that, in rats, cyclophosphamide (CPA) pretreatment increased infection of brain tumors by an intra-arterially administered herpes simplex virus type 1 OV, because it inhibited activation of complement responses, mediated by innate IgM. We also have previously shown that other pharmacologic inhibitors of complement, such as cobra venom factor (CVF), allowed for increased infection. However, in these studies, further inhibition of complement responses by CVF did not result in additional infection of brain tumor cells or in propagation of OV to surrounding tumor cells. In this study, we sought to determine if CPA did lead to increased infection/propagation from initially infected tumor cells. Unlike our results with CVF, we find that CPA administration does result in a time-dependent increase in infection of tumor cells, suggestive of increased propagation, in both syngeneic and athymic models of brain tumors. This increase was due to increased survival of OV within infected tumors and brain surrounding tumors. CPA's effect was not due to a direct enhancement of viral replication in tumor cells, rather was associated with its immunosuppressive effects. RT-PCR analysis revealed that CPA administration resulted in impaired mRNA production by peripheral blood mononuclear cells (PBMCs) of several cytokines (interferons alpha/beta, interferon gamma, TNFalpha, IL-15, and IL-18) with anti-HSV function. These findings suggest that the CPA-mediated facilitation of OV intraneoplastic propagation is associated with a general decrease of antiviral cytokines mRNAs in PBMCs. These findings not only suggest a potential benefit for the addition of transient immunosuppression in clinical applications of oncolytic HSV therapy, but also suggest that innate immunomodulatory pathways may be amenable to manipulation, in order to increase OV propagation and survival within infected tumors.
Figures






Similar articles
-
Complement depletion facilitates the infection of multiple brain tumors by an intravascular, replication-conditional herpes simplex virus mutant.J Virol. 2000 May;74(10):4765-75. doi: 10.1128/jvi.74.10.4765-4775.2000. J Virol. 2000. PMID: 10775615 Free PMC article.
-
Cyclophosphamide enhances glioma virotherapy by inhibiting innate immune responses.Proc Natl Acad Sci U S A. 2006 Aug 22;103(34):12873-8. doi: 10.1073/pnas.0605496103. Epub 2006 Aug 14. Proc Natl Acad Sci U S A. 2006. PMID: 16908838 Free PMC article.
-
Effects of innate immunity on herpes simplex virus and its ability to kill tumor cells.Gene Ther. 2003 Jun;10(11):983-90. doi: 10.1038/sj.gt.3302038. Gene Ther. 2003. PMID: 12756419 Review.
-
Oncolytic virus therapy of multiple tumors in the brain requires suppression of innate and elicited antiviral responses.Nat Med. 1999 Aug;5(8):881-7. doi: 10.1038/11320. Nat Med. 1999. PMID: 10426310
-
Oncolytic virus therapy for malignant gliomas: entering the new era.Expert Opin Biol Ther. 2023 Mar;23(3):269-282. doi: 10.1080/14712598.2023.2184256. Epub 2023 Mar 2. Expert Opin Biol Ther. 2023. PMID: 36809883 Review.
Cited by
-
Combinatorial strategies for oncolytic herpes simplex virus therapy of brain tumors.CNS Oncol. 2013 Mar;2(2):129-42. doi: 10.2217/cns.12.42. CNS Oncol. 2013. PMID: 23687568 Free PMC article. Review.
-
Oncolytic herpes viruses, chemotherapeutics, and other cancer drugs.Oncolytic Virother. 2013 Dec 4;2:57-74. doi: 10.2147/OV.S52601. eCollection 2013. Oncolytic Virother. 2013. PMID: 27512658 Free PMC article. Review.
-
Oncolytic herpes simplex virus and immunotherapy.BMC Immunol. 2018 Dec 18;19(1):40. doi: 10.1186/s12865-018-0281-9. BMC Immunol. 2018. PMID: 30563466 Free PMC article. Review.
-
Oncolytic herpes simplex virus-based strategies: toward a breakthrough in glioblastoma therapy.Front Microbiol. 2014 Jun 20;5:303. doi: 10.3389/fmicb.2014.00303. eCollection 2014. Front Microbiol. 2014. PMID: 24999342 Free PMC article. Review.
-
Herpes Simplex Oncolytic Viral Therapy for Malignant Glioma and Mechanisms of Delivery.World Neurosurg. 2025 Feb;194:123595. doi: 10.1016/j.wneu.2024.123595. Epub 2025 Jan 23. World Neurosurg. 2025. PMID: 39710201 Free PMC article. Review.
References
-
- Chiocca EA, Smith ER. Oncolytic viruses as novel anticancer agents: turning one scourge against another (In Process Citation) Expert Opin Investig Drugs. 2000;9:311–327. (MEDLINE record in process) - PubMed
-
- Kirn D, Martuza RL, Zwiebel J. Replication-selective virotherapy for cancer: biological principles, risk management and future directions. Nat Med. 2001;7:781–787. - PubMed
-
- Chiocca EA. Oncolytic viruses. Nat Rev Cancer. 2002;2:938–950. - PubMed
-
- Markert JM, et al. Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial (see comments) Gene Therapy. 2000;7:867–874. - PubMed
-
- Rampling R, et al. Toxicity evaluation of replication-competent herpes simplex virus (ICP 34.5 null mutant 1716) in patients with recurrent malignant glioma. Gene Therapy. 2000;7:859–866. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

