Hepatitis C genotyping by denaturing high-performance liquid chromatography
- PMID: 14715747
- PMCID: PMC321670
- DOI: 10.1128/JCM.42.1.158-163.2004
Hepatitis C genotyping by denaturing high-performance liquid chromatography
Abstract
Determination of the hepatitis C virus (HCV) genotype for infected patients increasingly has become accepted as the standard of care. Genotype assignment helps in assessing disease prognosis and assists in establishing the appropriate duration of treatment. The great genetic diversity of HCV, with 11 major genotypes and >70 subtypes, contributes to the technical difficulty of genotype testing. While the "gold standard" for testing is nucleic acid sequencing, a variety of hybridization assays, including the line probe assay, have been developed to provide more rapid and accessible forms of testing. The aim of this study was to determine whether denaturing high-performance liquid chromatography (dHPLC) could be used as a clinical method for distinguishing HCV genotypes 1, 2, 3, and 4. A portion of the 5' untranslated region of the HCV genome was amplified by heminested multiplex reverse transcription PCR. The two amplicons then were analyzed by dHPLC analysis and compared to the genotypes determined by sequence analysis. After 115 specimens were analyzed as standards, 200 masked specimens (specimens whose identity was not known before testing) were analyzed to determine the concordance of the assay. The assay had a concordance of 96% at the genotype level and a concordance of 87% at the subtype level. However, the dHPLC method was not as accurate as other reported methods of HCV genotyping. This is the first time that HCV genotyping has been performed by dHPLC.
Figures
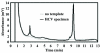
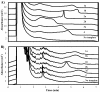

References
-
- Akuta, N., F. Suzuki, A. Tsubota, Y. Suzuki, T. Hosaka, T. Someya, M. Kobayashi, S. Saitoh, Y. Arase, K. Ikeda, and H. Kumada. 2003. Association of amino acid substitution pattern in nonstructural protein 5A of hepatitis C virus genotype2a low viral load and response to interferon monotherapy. J. Med. Virol. 69:376-383. - PubMed
-
- Bullock, G. C., D. E. Bruns, and D. M. Haverstick. 2002. Hepatitis C genotype determination by melting curve analysis with a single set of fluorescence resonance energy transfer probes. Clin. Chem. 48:2147-2154. - PubMed
-
- Di Bisceglie, A. M., and J. H. Hoofnagle. 2002. Optimal therapy of hepatitis C. Hepatology 36:S121-S127. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

