Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses
- PMID: 14715944
- PMCID: PMC6729585
- DOI: 10.1523/JNEUROSCI.4336-03.2004
Transient electrical coupling delays the onset of chemical neurotransmission at developing synapses
Abstract
The formation and subsequent elimination of electrical coupling between neurons has been demonstrated in many developing vertebrate and invertebrate nervous systems. The relationship between the disappearance of electrical synaptic connectivity and the appearance of chemical neurotransmission is not well understood. We report here that identified motoneurons from the snail Helisoma formed transient electrical and chemical connections during regeneration both in vivo and in vitro. Electrical connections that formed in vivo were strongest by day 2 and no longer detectable by day 7. During elimination of this electrical connection, an inhibitory chemical connection from 110 onto 19 formed. This sequence of synaptic development was recapitulated in cell culture with a similar time course. The relationship between the appearance of transient electrical coupling and its possible effects on the subsequent chemical synaptogenesis were examined by reducing transient intercellular coupling. Trophic factor-deprived medium resulted in a 66% reduction in coupling coefficient. In these conditions, the unidirectional chemical connection formed readily; in contrast, chemical synaptogenesis was delayed in cell pairs exposed to trophic factors where transient electrical coupling was strong. Dye coupling and synaptic vesicle cycling studies supported electrophysiological results. Exposure to cholinergic antagonists, curare and hexamethonium bromide, which block chemical neurotransmission in these synapses, resulted in prolonged maintenance of the electrical connection. These studies demonstrated an inverse relationship between chemical and electrical connectivity at early stages of synaptic development and suggest a dynamic interaction between these forms of neuronal communication as adult neural networks are constructed or regenerated.
Figures
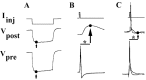

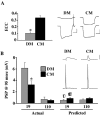
 p < 0.005;
p < 0.005;  p < 0.005), demonstrating that these voltage changes due to electrical coupling alone did not account for voltage differences at 95 msec post AP. Inset, Traces show a PSP in neuron 19 in response to an evoked AP in neuron 110 in either DM or CM. Horizontal bar equals 95 msec, vertical bar equals 20 mV for presynaptic (bottom) traces, 10 mV for postsynaptic (top) traces.
p < 0.005), demonstrating that these voltage changes due to electrical coupling alone did not account for voltage differences at 95 msec post AP. Inset, Traces show a PSP in neuron 19 in response to an evoked AP in neuron 110 in either DM or CM. Horizontal bar equals 95 msec, vertical bar equals 20 mV for presynaptic (bottom) traces, 10 mV for postsynaptic (top) traces.
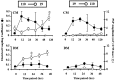
 p < 0.002). Left, bottom, Time course of synapse formation in DM. In the absence of trophic factors, electrical coupling never developed strongly, and ECC values were significantly lower than those in CM at 24 hr (*p < 0.0001). At this same time point, significantly higher PSP amplitudes were seen in DM than in CM (
p < 0.002). Left, bottom, Time course of synapse formation in DM. In the absence of trophic factors, electrical coupling never developed strongly, and ECC values were significantly lower than those in CM at 24 hr (*p < 0.0001). At this same time point, significantly higher PSP amplitudes were seen in DM than in CM ( p < 0.05). By 48 hr of contact in DM, ECC values were still low, but PSP amplitudes (8.7 ± 1.3 mV) had increased to values similar to those seen after 5d of culture in CM (CM at 120 hr: 9.0 ± 1.5 mV). Right, top and bottom, Time course of electrical and chemical synapse formation with current injection into neuron 19. ECCs were similar to 110 current injections with strong transient coupling in CM, but not DM. Thus, electrical synaptic connections were non-rectifying in both DM and CM pairs (right vs left graphs of ECC values). PSPs in neuron 110 at 95 msec from AP peak were virtually undetectable in response to stimulation of neuron 19 in both DM and CM. (n: DM, 0 hr = 16, 12 hr = 4, 24 hr = 16, 36 hr = 9, 48 hr = 7; CM, 0 hr = 15, 12 hr = 6, 24 hr = 32, 36 hr = 9, 48 hr = 6, 120 hr = 19.)
p < 0.05). By 48 hr of contact in DM, ECC values were still low, but PSP amplitudes (8.7 ± 1.3 mV) had increased to values similar to those seen after 5d of culture in CM (CM at 120 hr: 9.0 ± 1.5 mV). Right, top and bottom, Time course of electrical and chemical synapse formation with current injection into neuron 19. ECCs were similar to 110 current injections with strong transient coupling in CM, but not DM. Thus, electrical synaptic connections were non-rectifying in both DM and CM pairs (right vs left graphs of ECC values). PSPs in neuron 110 at 95 msec from AP peak were virtually undetectable in response to stimulation of neuron 19 in both DM and CM. (n: DM, 0 hr = 16, 12 hr = 4, 24 hr = 16, 36 hr = 9, 48 hr = 7; CM, 0 hr = 15, 12 hr = 6, 24 hr = 32, 36 hr = 9, 48 hr = 6, 120 hr = 19.)
References
-
- Aberg ND, Carlsson B, Rosengren L, Oscarsson J, Isaksson OG, Ronnback L, Eriksson PS (2000) Growth hormone increases connexin-43 expression in the cerebral cortex and hypothalamus. Endocrinology 141: 3879-3886. - PubMed
-
- Barker DL, Wong RG, Kater SB (1982) Separate factors produced by the CNS of the snail Helisoma stimulate neurite outgrowth and choline metabolism in cultured neurons. J Neurosci Res 8: 419-432. - PubMed
-
- Bem T, Le Feuvre Y, Simmers J, Meyrand P (2002) Electrical coupling can prevent expression of adult-like properties in an embryonic neural circuit. J Neurophysiol 87: 538-547. - PubMed
-
- Bennett MV (1997) Gap junctions as electrical synapses. J Neurocytol 26: 349-366. - PubMed
-
- Bennett MVL (1977) Electrical transmission: a functional analysis and comparison to chemical transmission. In: Handbook of physiology (Kandel ER, ed), pp 357-416. Bethesda: American Physiological Society.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
