Risks and benefits of preexposure and postexposure smallpox vaccination
- PMID: 14718077
- PMCID: PMC3035543
- DOI: 10.3201/eid0911.030369
Risks and benefits of preexposure and postexposure smallpox vaccination
Abstract
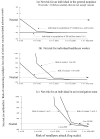
This article presents a model and decision criteria for evaluating a person's risk of pre- or postexposure smallpox vaccination in light of serious vaccine-related adverse events (death, postvaccine encephalitis and progressive vaccinia). Even at a 1-in-10 risk of 1,000 initial smallpox cases, a person in a population of 280 million has a greater risk for serious vaccine-related adverse events than a risk for smallpox. For a healthcare worker to accept preexposure vaccination, the risk for contact with an infectious smallpox case-patient must be >1 in 100, and the probability of 1,000 initial cases must be >1 in 1,000. A member of an investigation team would accept preexposure vaccination if his or her anticipated risk of contact is 1 in 2.5 and the risk of attack is assumed to be >1 in 16,000. The only circumstances in which postexposure vaccination would not be accepted are the following: if vaccine efficacy were <1%, the risk of transmission were <1%, and (simultaneously) the risk for serious vaccine-related adverse events were >1 in 5,000.
Figures


References
-
- Meltzer MI. Model 1. In: Institute of Medicine. Scientific and policy considerations in developing smallpox vaccination options: a workshop report. Washington: National Academies Press; 2002. p. 15–7. - PubMed
-
- Alibek K. Biohazard. New York: Random House; 1999.
-
- Neff JM. The case for abolishing routine childhood smallpox vaccination in the United States. Am J Epidemiol. 1971;93:245–7. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
