Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells
- PMID: 14718256
- PMCID: PMC1574216
- DOI: 10.1038/sj.bjp.0705633
Octreotide regulates CC but not CXC LPS-induced chemokine secretion in rat Kupffer cells
Abstract
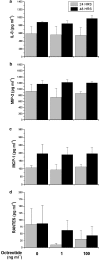
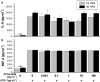
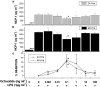
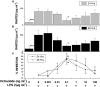
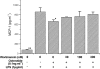
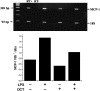
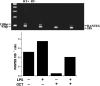
Kupffer cells (KC) and lipopolysaccharide (LPS) interaction is the initial event leading to hepatic inflammation and fibrosis in many types of liver injury. We studied chemokine secretion by KC activated with LPS and the possible effect of the somatostatin analogue octreotide, in the regulation of this process. KC isolated from Sprague-Dawley rats were cultured in the presence of LPS added alone or with different concentrations of octreotide for 24 and 48 h, and chemokine production was assessed in culture supernatants by ELISA. CC chemokine mRNA expression was assessed by semiquantitative RT-PCR. Vehicle-stimulated KC produced a basal amount of CC and CXC chemokines. LPS-stimulated KC secreted significantly increased amounts of IL-8 (GRO/CINC-1) (P<0.001), MIP-2 (P<0.001), MCP-1 (P<0.001), and RANTES (P<0.01). Octreotide inhibited LPS-induced secretion of the CC chemokines MCP-1 (P<0.05) and RANTES (P<0.05), but not the CXC chemokines IL-8 (GRO/CINC-1) and MIP-2, in a concentration-dependent manner. Downregulation of basal and LPS-induced mRNA expression of the CC chemokines was also observed in the presence of octreotide. Pretreatment with phosphatidylinositol 3 (PI3)-kinase inhibitors reduced chemokine production by LPS-treated KC in both the mRNA and protein level. Furthermore, it prevented the octreotide inhibitory effect on LPS-induced chemokine secretion, indicating a possible involvement of the PI3-kinase pathway. In conclusion, these data demonstrate that chemokine secretion by KC can be differentially regulated by octreotide, and suggest that this somatostatin analogue may have immunoregulatory effects on resident liver macrophages. British Journal of Pharmacology (2004) 141, 477-487. doi:10.1038/sj.bjp.0705633
Figures



References
-
- ANDOH A., HATA K., SHIMADA M., FUJINO S., TASAKI K., BAMBA S., ARAKI Y., FUJIYAMA Y., BAMBA T. Inhibitory effects of somatostatin on tumor necrosis factor-alpha-induced interleukin-6 secretion in human pancreatic periacinar myofibroblasts. Int. J. Mol. Med. 2002;10:89–93. - PubMed
-
- BAYON L.G., IZQUIERDO M.A., SIROVICH I., VAN ROOIJEN N., BEELEN R.H., MEIJER S. Role of Kupffer cells in arresting circulating tumor cells and controlling metastatic growth in the liver. Hepatology. 1996;23:1224–1231. - PubMed
-
- BONE-LARSON C.L., SIMPSON K.J., COLLETTI L.M., LUKACS N.W., CHEN S.C., LIRA S., KUNKEL S.L., HOGABOAM C.M. The role of chemokines in the immunopathology of the liver. Immunol. Rev. 2000;177:8–20. - PubMed
-
- BUKARA M., BAUTISTA A.P. Acute alcohol intoxication and gadolinium chloride attenuate endotoxin-induced release of CC chemokines in the rat. Alcohol. 2000;20:193–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

