Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body
- PMID: 14718520
- PMCID: PMC2172340
- DOI: 10.1083/jcb.200308132
Intraflagellar transport (IFT) cargo: IFT transports flagellar precursors to the tip and turnover products to the cell body
Erratum in
- J Cell Biol. 2004 Mar 15;164(6):following 941
Abstract
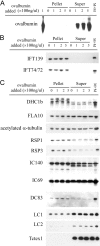
Intraflagellar transport (IFT) is the bidirectional movement of multisubunit protein particles along axonemal microtubules and is required for assembly and maintenance of eukaryotic flagella and cilia. One posited role of IFT is to transport flagellar precursors to the flagellar tip for assembly. Here, we examine radial spokes, axonemal subunits consisting of 22 polypeptides, as potential cargo for IFT. Radial spokes were found to be partially assembled in the cell body, before being transported to the flagellar tip by anterograde IFT. Fully assembled radial spokes, detached from axonemal microtubules during flagellar breakdown or turnover, are removed from flagella by retrograde IFT. Interactions between IFT particles, motors, radial spokes, and other axonemal proteins were verified by coimmunoprecipitation of these proteins from the soluble fraction of Chlamydomonas flagella. These studies indicate that one of the main roles of IFT in flagellar assembly and maintenance is to transport axonemal proteins in and out of the flagellum.
Figures




References
-
- Bell, S.E., A. Mavila, R. Salazar, K.J. Bayless, S. Kanagala, S.A. Maxwell, and G.E. Davis. 2001. Differential gene expression during capillary morphogenesis in 3D collagen matrices: regulated expression of genes involved in basement membrane matrix assembly, cell cycle progression, cellular differentiation and G-protein signaling. J. Cell Sci. 114:2755–2773. - PubMed
-
- Benashski, S.E., R.S. Patel-King, and S.M. King. 1999. Light chain 1 from the Chlamydomonas outer dynein arm is a leucine-rich repeat protein associated with the motor domain of the heavy chain. Biochemistry. 38:7253–7264. - PubMed
-
- Brazelton, W., C. Amundsen, C. Silflow, and P. Lefebvre. 2001. The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr. Biol. 11:1591–1594. - PubMed
-
- Cole, D.G. 2003. The intraflagellar transport machinery of Chlamydomonas reinhardtii. Traffic. 4:435–442. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

