Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion
- PMID: 14718567
- PMCID: PMC363054
- DOI: 10.1091/mbc.e03-07-0528
Fibronectin matrix assembly regulates alpha5beta1-mediated cell cohesion
Abstract
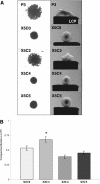
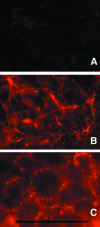
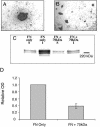
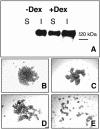
Integrin-extracellular matrix (ECM) interactions in two-dimensional (2D) culture systems are widely studied (Goldstein and DiMilla, 2002. J Biomed. Mater. Res. 59, 665-675; Koo et al., 2002. J. Cell Sci. 115, 1423-1433). Less understood is the role of the ECM in promoting intercellular cohesion in three-dimensional (3D) environments. We have demonstrated that the alpha5beta1-integrin mediates strong intercellular cohesion of 3D cellular aggregates (Robinson et al., 2003. J. Cell Sci. 116, 377-386). To further investigate the mechanism of alpha5beta1-mediated cohesivity, we used a series of chimeric alpha5beta1-integrin-expressing cells cultured as multilayer cellular aggregates. In these cell lines, the alpha5 subunit cytoplasmic domain distal to the GFFKR sequence was truncated, replaced with that of the integrin alpha4, the integrin alpha2, or maintained intact. Using these cells, alpha5beta1-integrin-mediated cell aggregation, compaction and cohesion were determined and correlated with FN matrix assembly. The data presented demonstrate that cells cultured in the absence of external mechanical support can assemble a FN matrix that promotes integrin-mediated aggregate compaction and cohesion. Further, inhibition of FN matrix assembly blocks the intercellular associations required for compaction, resulting in cell dispersal. These results demonstrate that FN matrix assembly contributes significantly to tissue cohesion and represents an alternative mechanism for regulating tissue architecture.
Figures



References
-
- Adamska, M., MacDonald, B.T., and Meisler, M.H. (2003). Doubleridge, a mouse mutant with defective compaction of the apical ectodermal ridge and normal dorsal-ventral patterning of the limb. Dev. Biol. 255, 350-362. - PubMed
-
- Akamatsu, H., Ichihara-Tanaka, K., Ozono, K., Kamiike, W., Matsuda, H., and Sekiguchi, K. (1996). Suppression of transformed phenotypes of human fibrosarcoma cells by overexpression of recombinant fibronectin. Cancer Res. 56, 4541-4546. - PubMed
-
- Akiyama, S.K., Aota, S., and Yamada, K.M. (1995). Function and receptor specificity of a minimal 20 kilodalton cell adhesive fragment of fibronectin. Cell Adhes. Commun. 3, 13-25. - PubMed
-
- Armstrong, P.B., and Armstrong, M.T. (2000). Intercellular invasion and the organizational stability of tissues: a role for fibronectin. Biochim. Biophys. Acta 1470, O9-O20. - PubMed
-
- Awad, H.A., Boivin, G.P., Dressler, M.R., Smith, F.N., Young, R.G., and Butler, D.L. (2003). Repair of patellar tendon injuries using a cell-collagen composite. J. Orthop. Res. 21, 420-431. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

