A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells
- PMID: 14718571
- PMCID: PMC363138
- DOI: 10.1091/mbc.e03-05-0296
A polycystin-1 multiprotein complex is disrupted in polycystic kidney disease cells
Abstract
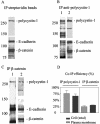
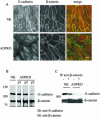
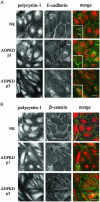
Autosomal dominant polycystic kidney disease (ADPKD) is typified by the accumulation of fluid-filled cysts and abnormalities in renal epithelial cell function. The disease is principally caused by mutations in the gene encoding polycystin-1, a large basolateral plasma membrane protein expressed in kidney epithelial cells. Our studies reveal that, in normal kidney cells, polycystin-1 forms a complex with the adherens junction protein E-cadherin and its associated catenins, suggesting a role in cell adhesion or polarity. In primary cells from ADPKD patients, the polycystin-1/polycystin-2/E-cadherin/beta-catenin complex was disrupted and both polycystin-1 and E-cadherin were depleted from the plasma membrane as a result of the increased phosphorylation of polycystin-1. The loss of E-cadherin was compensated by the transcriptional upregulation of the normally mesenchymal N-cadherin. Increased cell surface N-cadherin in the disease cells in turn stabilized the continued plasma membrane localization of beta-catenin in the absence of E-cadherin. The results suggest that enhanced phosphorylation of polycystin-1 in ADPKD cells precipitates changes in its localization and its ability to form protein complexes that are critical for the stabilization of adherens junctions and the maintenance of a fully differentiated polarized renal epithelium.
Figures
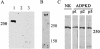



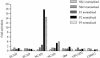
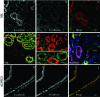
References
-
- Angst, B.D., Marcozzi, C., and Magee, A.I. (2001). The cadherin superfamily: diversity in form and function. J. Cell Sci. 114, 629-641. - PubMed
-
- Arnaout, M.A. (2001). Molecular genetics and pathogenesis of autosomal dominant polycystic kidney disease. Annu. Rev. Med. 52, 93-123. - PubMed
-
- Arnould, T., Kim, E., Tsiokas, L., Jochimsen, F., Gruning, W., Chang, J.D., and Walz, G. (1998). The polycystic kidney disease 1 gene product mediates protein kinase C alpha-dependent and c-Jun N-terminal kinase-dependent activation of the transcription factor AP-1. J. Biol. Chem. 273, 6013-6018. - PubMed
-
- Balsamo, J., and Lilien, J. (1990). N-cadherin is stably associated with and is an acceptor for a cell surface N-acetylgalactosaminylphosphotransferase. J. Biol. Chem. 265, 2923-2928. - PubMed
-
- Barbar, E., Rola-Pleszczynski, M., Payet, M.D., and Dupuis, G. (2003). Protein kinase C inhibits the transplasma membrane influx of Ca2+ triggered by 4-aminopyridine in Jurkat T lymphocytes. Biochim. Biophys. Acta 1622, 89-98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

