MLL 5 protein forms intranuclear foci, and overexpression inhibits cell cycle progression
- PMID: 14718661
- PMCID: PMC321754
- DOI: 10.1073/pnas.2036345100
MLL 5 protein forms intranuclear foci, and overexpression inhibits cell cycle progression
Abstract
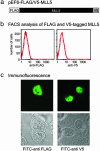
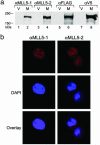
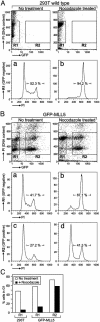
MLL5 is a mammalian trithorax group (trx-G) gene identified within chromosome band 7q22, a frequently deleted element found in cytogenetic aberrations of acute myeloid malignancies. MLL5 cDNA was linked with the FLAG and V5 tags at the N and C terminus, respectively, and transfected into 293T cells. Immunofluoresence staining of the expressed tagged MLL5 protein showed localization to the nucleus and exclusion from nucleoli, and no surface staining was detected. Both ectopically introduced and endogenous MLL5 protein displayed a speckled nuclear distribution. By using a series of MLL5-truncated mutants fused with enhanced GFP, a domain (residues 945-1,156) required for foci accumulation was identified, and regions containing functional nuclear localization signals were mapped. Ectopic overexpression of GFP-MLL5 induced cell cycle arrest in G(1) phase. This inhibition of cell cycle progression was indicated by delayed progression into nocodazole-induced mitotic arrest and was confirmed by a lack of BrdUrd incorporation. These findings suggest that MLL5 forms intranuclear protein complexes that may play an important role in chromatin remodeling and cellular growth suppression.
Figures

References
-
- Gu, Y., Nakamura, T., Alder, H., Prasad, R., Canaani, O., Cimino, G., Croce, C. M. & Canaani, E. (1992) Cell 71, 701–708. - PubMed
-
- Tkachuk, D. C., Kohler, S. & Cleary, M. L. (1992) Cell 71, 691–700. - PubMed
-
- Djabali, M., Selleri, L., Parry, P., Bower, M., Young, B. D. & Evans, G. A. (1992) Nat. Genet. 2, 113–118. - PubMed
-
- Dimartino, J. F. & Cleary, M. L. (1999) Br. J. Haematol. 106, 614–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

