TGF-beta, T-cell tolerance and anti-CD3 therapy
- PMID: 14720578
- PMCID: PMC2796480
- DOI: 10.1016/j.molmed.2003.11.007
TGF-beta, T-cell tolerance and anti-CD3 therapy
Abstract
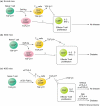
Recent studies by Chatenoud and co-workers suggest that non-mitogenic F(ab′)2 fragments of anti-CD3 antibodies, which cannot bind the Fc receptor, induce a prolonged period of tolerance and prevent diabetes in nonobese diabetic (NOD) mice. Tolerance is established by regulatory T cells through the production of transforming growth factor-β1 (TGF-β1), but the mechanism by which TGF-β1 confers this activity is unclear. Analysis of mice deficient in TGF-β1 suggests that TGF-β1 raises the threshold at which intracellular calcium activates T cells to a level that prevents an autoimmune response.
Figures

References
-
- Belghith M, et al. TGF-β-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 2003;9:1202–1208. - PubMed
-
- Stockinger B. T lymphocyte tolerance: from thymic deletion to peripheral control mechanisms. Adv. Immunol. 1999;71:229–265. - PubMed
-
- Bonomo A, et al. Premature escape of double-positive thymocytes to the periphery of young mice. Possible role in autoimmunity. J. Immunol. 1994;152:1509–1514. - PubMed
-
- Wood KJ, Sakaguchi S. Regulatory T cells in transplantation tolerance. Nat. Rev. Immunol. 2003;3:199–210. - PubMed
-
- Shevach EM. CD4+CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2002;2:389–400. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

