Dual effects of 17beta-oestradiol on interleukin 1beta-induced proteoglycan degradation in chondrocytes
- PMID: 14722210
- PMCID: PMC1754890
- DOI: 10.1136/ard.2003.006510
Dual effects of 17beta-oestradiol on interleukin 1beta-induced proteoglycan degradation in chondrocytes
Abstract
Objective: To determine whether 17beta-oestradiol (E2) modulates interleukin (IL) 1beta-induced proteoglycan degradation in chondrocytes, and to analyse the part played by metalloproteinases (MMPs) in this process.
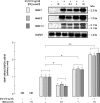
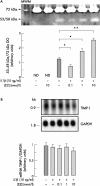
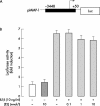
Methods: Primary cultured rabbit articular chondrocytes were prepared and treated with 10 ng/ml IL1beta combined or not with 0.1-10 nM E2. Neosynthesised proteoglycans (PGs) were evaluated after incorporation of [(35)SO(4)]sulphate and further analysed after chromatography on a Sepharose 2B column. Chondrocyte mRNA levels of aggrecan, MMP-1, -3, -13, and tissue inhibitor of metalloproteinase-1 (TIMP-1) were studied by northern blot. MMP-1 activity was measured by zymography. MMP-1 gene transcription was studied by transient transfection of chondrocytes with an MMP-1-luciferase construct.
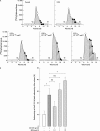
Results: E2 modulated the IL1beta-induced total sulphated PGs in rabbit articular chondrocytes, which decreased as the E2 concentration was increased. At a low concentration (0.1 nmol/l) E2 counteracts the IL1beta-induced decrease in sulphated PG, while at high concentration (10 nmol/l) E2 enhances the IL1beta effects. A biphasic E2 effect was also observed on IL1beta-induced disaggregation of PG, 53-58 kDa gelatinolytic activity, and MMP-1, -3, and -13 mRNA levels. In contrast, E2 did not modify the level of aggrecan mRNA and had no effect on TIMP-1 mRNA expression. Finally, simultaneous addition of IL1beta and E2 (0.1-10 nmol/l) did not modify IL1beta-induced MMP-1-luciferase activity, suggesting that E2 effects probably occur at the post-transcriptional level of MMP gene expression.
Conclusion: Oestrogen concentration may have an inverse effect on IL1beta stimulated proteoglycan degradation and MMP production by chondrocytes.
Figures

Similar articles
-
Inhibition of activator protein 1 activity by paclitaxel suppresses interleukin-1-induced collagenase and stromelysin expression by bovine chondrocytes.Arthritis Rheum. 1998 May;41(5):869-76. doi: 10.1002/1529-0131(199805)41:5<869::AID-ART15>3.0.CO;2-3. Arthritis Rheum. 1998. PMID: 9588740
-
Mechanotransduction via integrins and interleukin-4 results in altered aggrecan and matrix metalloproteinase 3 gene expression in normal, but not osteoarthritic, human articular chondrocytes.Arthritis Rheum. 2000 Sep;43(9):2091-9. doi: 10.1002/1529-0131(200009)43:9<2091::AID-ANR21>3.0.CO;2-C. Arthritis Rheum. 2000. PMID: 11014361
-
Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes.J Biochem. 1998 Mar;123(3):431-9. doi: 10.1093/oxfordjournals.jbchem.a021955. J Biochem. 1998. PMID: 9538225
-
Glucosamine sulfate modulates the levels of aggrecan and matrix metalloproteinase-3 synthesized by cultured human osteoarthritis articular chondrocytes.Osteoarthritis Cartilage. 2003 Jun;11(6):424-32. doi: 10.1016/s1063-4584(03)00052-9. Osteoarthritis Cartilage. 2003. PMID: 12801482
-
Effects of proinflammatory cytokines on canine articular chondrocytes in a three-dimensional culture.Am J Vet Res. 2005 Jul;66(7):1187-96. doi: 10.2460/ajvr.2005.66.1187. Am J Vet Res. 2005. PMID: 16111157
Cited by
-
Sex-dependent variation in cartilage adaptation: from degeneration to regeneration.Biol Sex Differ. 2023 Apr 5;14(1):17. doi: 10.1186/s13293-023-00500-3. Biol Sex Differ. 2023. PMID: 37024929 Free PMC article. Review.
-
Menopause and Rheumatic Disease.Rheum Dis Clin North Am. 2017 May;43(2):287-302. doi: 10.1016/j.rdc.2016.12.011. Rheum Dis Clin North Am. 2017. PMID: 28390570 Free PMC article. Review.
-
Cepharanthine Ameliorates Chondrocytic Inflammation and Osteoarthritis via Regulating the MAPK/NF-κB-Autophagy Pathway.Front Pharmacol. 2022 Jun 21;13:854239. doi: 10.3389/fphar.2022.854239. eCollection 2022. Front Pharmacol. 2022. PMID: 35800437 Free PMC article.
-
Relationship between soy milk intake and radiographic knee joint space narrowing and osteophytes.Rheumatol Int. 2016 Sep;36(9):1215-22. doi: 10.1007/s00296-016-3491-6. Epub 2016 May 18. Rheumatol Int. 2016. PMID: 27193467
-
Pulse electromagnetic fields effects on serum E2 levels, chondrocyte apoptosis, and matrix metalloproteinase-13 expression in ovariectomized rats.Rheumatol Int. 2009 Jun;29(8):927-35. doi: 10.1007/s00296-008-0782-6. Epub 2008 Dec 3. Rheumatol Int. 2009. PMID: 19050895
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

