The membrane-proximal tyrosine-based sorting signal of human immunodeficiency virus type 1 gp41 is required for optimal viral infectivity
- PMID: 14722262
- PMCID: PMC321364
- DOI: 10.1128/jvi.78.3.1069-1079.2004
The membrane-proximal tyrosine-based sorting signal of human immunodeficiency virus type 1 gp41 is required for optimal viral infectivity
Abstract
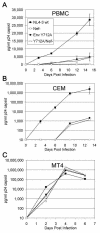
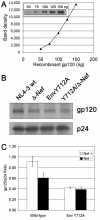
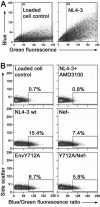
The membrane-proximal tyrosine-based sorting motif in the cytoplasmic domain of the human immunodeficiency virus type 1 Env glycoprotein is important for endocytosis from the plasma membrane, basolateral targeting of viral budding in polarized epithelial cells, and polarized budding from a localized region of the lymphocyte plasma membrane. To study the role of the Env sorting motif (Y712XXL) in infectivity, the incorporation of Env into virions, and viral entry, we disrupted the motif with a tyrosine-to-alanine substitution. To investigate the relationship between the Env sorting motif and the enhancement of infectivity by Nef, the EnvY712A substitution was made in both Nef-positive and Nef-negative backgrounds. In spreading infections, including those using primary lymphocytes, the growth of the Y712A mutant was as impaired as Nef-negative virus, and the EnvY712A/Delta-Nef combination mutant was almost completely defective. In single-round infections using CD4-positive HeLa cells, the EnvY712A mutation impaired infectivity, and Nef retained the ability to enhance the infectivity in the context of EnvY712A. EnvY712 and Nef were required for the optimal infectivity of virions produced from either HEK293T or MT4 cells, but these sequences were required for the optimal incorporation of Env only when virions were produced from MT4 cells. Despite the wild-type levels of Env in viruses produced from 293T cells, the entry of the EnvY712A and Delta-Nef mutants into target cells was impaired. We conclude that the membrane-proximal tyrosine-based sorting motif of gp41 Env is, like Nef, important for optimal viral infectivity and, in the case of MT4 T cells, virion incorporation of Env. Nef does not require the Y712XXL motif to enhance viral infectivity. The finding that EnvY712 and Nef each affect the efficiency of viral entry independently of the Env content of virions suggests that both viral proteins are involved in trafficking events that influence morphogenesis to produce maximally fusogenic virus.
Figures
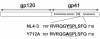




References
-
- Berlioz-Torrent, C., B. L. Shacklett, L. Erdtmann, L. Delamarre, I. Bouchaert, P. Sonigo, M. C. Dokhelar, and R. Benarous. 1999. Interactions of the cytoplasmic domains of human and simian retroviral transmembrane proteins with components of the clathrin adaptor complexes modulate intracellular and cell surface expression of envelope glycoproteins. J. Virol. 73:1350-1361. - PMC - PubMed
-
- Boge, M., S. Wyss, J. S. Bonifacino, and M. Thali. 1998. A membrane-proximal tyrosine-based signal mediates internalization of the HIV-1 envelope glycoprotein via interaction with the AP-2 clathrin adaptor. J. Biol. Chem. 273:15773-15778. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

