Simian immunodeficiency virus promoter exchange results in a highly attenuated strain that protects against uncloned challenge virus
- PMID: 14722263
- PMCID: PMC321388
- DOI: 10.1128/jvi.78.3.1080-1092.2004
Simian immunodeficiency virus promoter exchange results in a highly attenuated strain that protects against uncloned challenge virus
Abstract
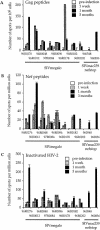
Among the many simian immunodeficiency virus (SIV) immunogens, only live attenuated viral vaccines have afforded strong protection to a natural pathogenic isolate. Since the promoter is crucial to the tempo of viral replication in general, it was reasoned that promoter exchange might confer a novel means of attenuating SIV. The core enhancer and promoter sequences of the SIV macaque 239nefstop strain (NF-kappaB/Sp1 region from -114 bp to mRNA start) have been exchanged for those of the human cytomegalovirus immediate-early promoter (CMV-IE; from -525 bp to mRNA start). During culture of the resulting virus, referred to as SIVmegalo, on CEMx174 or rhesus macaque peripheral blood mononuclear cells, deletions arose in distal regions of the CMV-IE sequences that stabilized after 1 or 2 months of culture. However, when the undeleted form of SIVmegalo was inoculated into rhesus macaques, animals showed highly controlled viremia during primary and persistent infection. Compared to parental virus infection in macaques, primary viremia was reduced by >1,000-fold to undetectable levels, with little sign of an increase of cycling cells in lymph nodes, CD4(+) depletion, or altered T-cell activation markers in peripheral blood. Moreover, in contrast to wild-type infection in most infected animals, the nef stop mutation did not revert to the wild-type codon, indicating yet again that replication was dramatically curtailed. Despite such drastic attenuation, antibody titers and enzyme-linked immunospot reactivity to SIV peptides, although slower to appear, were comparable to those seen in a parental virus infection. When animals were challenged intravenously at 4 or 6 months with the uncloned pathogenic SIVmac251 strain, viremia was curtailed by approximately 1,000-fold at peak height without any sign of hyperactivation in CD4(+)- or CD8(+)-T-cell compartment or increase in lymph node cell cycling. To date, there has been a general inverse correlation between attenuation and protection; however, these findings show that promoter exchange constitutes a novel means to highly attenuate SIV while retaining the capacity to protect against challenge virus.
Figures




References
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. - PubMed
-
- Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. - PubMed
-
- Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, M. G. Lewis, E. A. Emini, J. W. Shiver, and N. L. Letvin. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. - PubMed
-
- Blancou, P., N. Chenciner, M. C. Cumont, S. Wain-Hobson, B. Hurtrel, and R. Cheynier. 2001. The infiltration kinetics of simian immunodeficiency virus-specific T cells drawn to sites of high antigenic stimulation determines local in vivo viral escape. Proc. Natl. Acad. Sci. USA 98:13237-13242. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

