A nonproliferating parvovirus vaccine vector elicits sustained, protective humoral immunity following a single intravenous or intranasal inoculation
- PMID: 14722265
- PMCID: PMC321389
- DOI: 10.1128/jvi.78.3.1101-1108.2004
A nonproliferating parvovirus vaccine vector elicits sustained, protective humoral immunity following a single intravenous or intranasal inoculation
Abstract
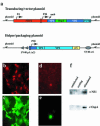
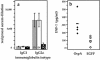
An ideal vaccine delivery system would elicit persistent protection following a single administration, preferably by a noninvasive route, and be safe even in the face of immunosuppression, either inherited or acquired, of the recipient. We have exploited the unique life cycle of the autonomous parvoviruses to develop a nonproliferating vaccine platform that appears to both induce priming and continually boost a protective immune response following a single inoculation. A crippled parvovirus vector was constructed, based on a chimera between minute virus of mice (MVM) and LuIII, which expresses Borrelia burgdorferi outer surface protein A (OspA) instead of its coat protein. The vector was packaged into an MVM lymphotropic capsid and inoculated into naive C3H/HeNcr mice. Vaccination with a single vector dose, either intravenously or intranasally, elicited high-titer anti-OspA-specific antibody that provided protection from live spirochete challenge and was sustained over the lifetime of the animal. Both humoral and cell-mediated Th(1) immunity was induced, as shown by anti-OspA immunoglobulin G2a antibody and preferential gamma interferon production by OspA-specific CD4(+) T cells.
Figures




Similar articles
-
Reservoir targeted vaccine for lyme borreliosis induces a yearlong, neutralizing antibody response to OspA in white-footed mice.Clin Vaccine Immunol. 2011 Nov;18(11):1809-16. doi: 10.1128/CVI.05226-11. Epub 2011 Sep 14. Clin Vaccine Immunol. 2011. PMID: 21918116 Free PMC article.
-
Protective efficacy of an oral vaccine to reduce carriage of Borrelia burgdorferi (strain N40) in mouse and tick reservoirs.Vaccine. 2006 Mar 10;24(11):1949-57. doi: 10.1016/j.vaccine.2005.10.044. Epub 2005 Nov 4. Vaccine. 2006. PMID: 16300863 Free PMC article.
-
Evaluation of OspA vaccination-induced serological correlates of protection against Lyme borreliosis in a mouse model.PLoS One. 2013 Nov 18;8(11):e79022. doi: 10.1371/journal.pone.0079022. eCollection 2013. PLoS One. 2013. PMID: 24260146 Free PMC article.
-
Relationship between immunity to Borrelia burgdorferi outer-surface protein A (OspA) and Lyme arthritis.Clin Infect Dis. 2011 Feb;52 Suppl 3(Suppl 3):s259-65. doi: 10.1093/cid/ciq117. Clin Infect Dis. 2011. PMID: 21217173 Free PMC article. Review.
-
A brief history of OspA vaccines including their impact on diagnostic testing for Lyme disease.Diagn Microbiol Infect Dis. 2022 Jan;102(1):115572. doi: 10.1016/j.diagmicrobio.2021.115572. Epub 2021 Oct 10. Diagn Microbiol Infect Dis. 2022. PMID: 34763193 Review.
Cited by
-
Blood safety and the choice of anti-hemophilic factor concentrate.Pediatr Blood Cancer. 2006 Sep;47(3):245-54. doi: 10.1002/pbc.20895. Pediatr Blood Cancer. 2006. PMID: 16724312 Free PMC article. Review.
-
Parvovirus B19 infection in human pregnancy.BJOG. 2011 Jan;118(2):175-86. doi: 10.1111/j.1471-0528.2010.02749.x. Epub 2010 Oct 13. BJOG. 2011. PMID: 21040396 Free PMC article. Review.
-
Parvoviral left-end hairpin ears are essential during infection for establishing a functional intranuclear transcription template and for efficient progeny genome encapsidation.J Virol. 2013 Oct;87(19):10501-14. doi: 10.1128/JVI.01393-13. Epub 2013 Jul 31. J Virol. 2013. PMID: 23903839 Free PMC article.
-
Parvoviral virions deploy a capsid-tethered lipolytic enzyme to breach the endosomal membrane during cell entry.Proc Natl Acad Sci U S A. 2005 Nov 22;102(47):17148-53. doi: 10.1073/pnas.0508477102. Epub 2005 Nov 11. Proc Natl Acad Sci U S A. 2005. PMID: 16284249 Free PMC article.
References
-
- Alexopoulou, L., V. Thomas, M. Schnare, Y. Lobet, J. Anguita, R. T. Schoen, R. Medzhitov, E. Fikrig, and R. A. Flavell. 2002. Hyporesponsiveness to vaccination with Borrelia burgdorferi OspA in humans and in TLR1- and TLR2-deficient mice. Nat. Med. 8:878-884. - PubMed
-
- Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2002. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Vaccine 20:1949-1955. - PubMed
-
- Armstrong, A. L., S. W. Barthold, D. H. Persing, and D. S. Beck. 1992. Carditis in Lyme disease susceptible and resistant strains of laboratory mice infected with Borrelia burgdorferi. Am. J. Trop. Med. Hyg. 47:249-258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

