Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins
- PMID: 14722266
- PMCID: PMC321412
- DOI: 10.1128/jvi.78.3.1109-1120.2004
Downregulation of major histocompatibility complex class I by human ubiquitin ligases related to viral immune evasion proteins
Abstract
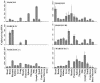
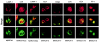
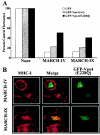
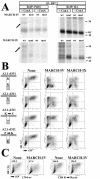
Poxviruses and gamma-2 herpesviruses share the K3 family of viral immune evasion proteins that inhibit the surface expression of glycoproteins such as major histocompatibility complex class I (MHC-I), B7.2, ICAM-1, and CD95(Fas). K3 family proteins contain an amino-terminal PHD/LAP or RING-CH domain followed by two transmembrane domains. To examine whether human homologues are functionally related to the viral immunoevasins, we studied seven membrane-associated RING-CH (MARCH) proteins. All MARCH proteins located to subcellular membranes, and several MARCH proteins reduced surface levels of known substrates of the viral K3 family. Two closely related proteins, MARCH-IV and MARCH-IX, reduced surface expression of MHC-I molecules. In the presence of MARCH-IV or MARCH-IX, MHC-I was ubiquitinated and rapidly internalized by endocytosis, whereas MHC-I molecules lacking lysines in their cytoplasmic tail were resistant to downregulation. The amino-terminal regions containing the RING-CH domain of several MARCH proteins examined catalyzed multiubiquitin formation in vitro, suggesting that MARCH proteins are ubiquitin ligases. The functional similarity of the MARCH family and the K3 family suggests that the viral immune evasion proteins were derived from MARCH proteins, a novel family of transmembrane ubiquitin ligases that seems to target glycoproteins for lysosomal destruction via ubiquitination of the cytoplasmic tail.
Figures





References
-
- Aasland, R., T. J. Gibson, and A. F. Stewart. 1995. The PHD finger: implications for chromatin-mediated transcriptional regulation. Trends Biochem. Sci. 20:56-59. - PubMed
-
- Aravind, L., L. M. Iyer, and E. V. Koonin. 2003. Scores of RINGS but no PHDs in ubiquitin signaling. Cell Cycle 2:123-126. - PubMed
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. - PubMed
-
- Biederer, T., C. Volkwein, and T. Sommer. 1997. Role of Cue1p in ubiquitination and degradation at the ER surface. Science 278:1806-1809. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

