Nuclear localization but not PML protein is required for incorporation of the papillomavirus minor capsid protein L2 into virus-like particles
- PMID: 14722267
- PMCID: PMC321415
- DOI: 10.1128/jvi.78.3.1121-1128.2004
Nuclear localization but not PML protein is required for incorporation of the papillomavirus minor capsid protein L2 into virus-like particles
Abstract
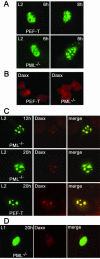
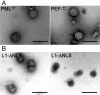
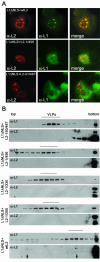
Recent reports suggest that nuclear domain(s) 10 (ND10) is the site of papillomavirus morphogenesis. The viral genome replicates in or close to ND10. In addition, the minor capsid protein, L2, accumulates in these subnuclear structures and recruits the major capsid protein, L1. We have now used cell lines deficient for promyelocytic leukemia (PML) protein, the main structural component of ND10, to study the role of this nuclear protein for L2 incorporation into virus-like particles (VLPs). L2 expressed in PML protein knockout (PML(-/-)) cells accumulated in nuclear dots, which resemble L2 aggregates forming at ND10 in PML protein-containing cells. These L2 assemblies also attracted L1 and the transcriptional repressor Daxx, suggesting that they are functional in the absence of PML protein. In addition, L2-containing VLPs assembled in PML(-/-) cells. In order to analyze whether incorporation of L2 into VLPs requires any specific subcellular localization, an L1 mutant defective for nuclear transport and L2 mutants deficient in nuclear translocation and/or ND10 localization were constructed. Using this approach, we identified two independent L2 domains interacting with L1. Mutant L2 proteins not accumulating in ND10 were incorporated into VLPs. Mutant L1 protein, which assembled into VLPs in the cytoplasm, did not incorporate L2 defective for nuclear translocation. The same mutant L2 protein, which passively diffuses into the nucleus, is incorporated into wild-type L1-VLPs in the nucleus. Our data demonstrate that the incorporation of L2 into VLPs requires nuclear but not ND10 localization.
Figures


References
-
- Becker, K. A., L. Florin, C. Sapp, and M. Sapp. 2003. Dissection of human papillomavirus type 33 L2 domains involved in nuclear domain (ND) 10 homing and reorganization. Virology 314:161-167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

