The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo
- PMID: 14722273
- PMCID: PMC321405
- DOI: 10.1128/jvi.78.3.1181-1194.2004
The immediate-early 63 protein of Varicella-Zoster virus: analysis of functional domains required for replication in vitro and for T-cell and skin tropism in the SCIDhu model in vivo
Abstract
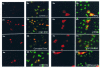
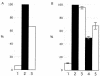
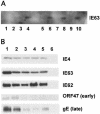
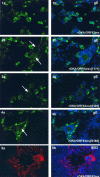
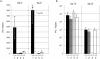
The immediate-early 63-kDa (IE63) protein of varicella-zoster virus (VZV) is a phosphoprotein encoded by open reading frame (ORF) ORF63/ORF70. To identify functional domains, 22 ORF63 mutations were evaluated for effects on IE63 binding to the major VZV transactivator, IE62, and on IE63 phosphorylation and nuclear localization in transient transfections, and after insertion into the viral genome with VZV cosmids. The IE62 binding site was mapped to IE63 amino acids 55 to 67, with R59/L60 being critical residues. Alanine substitutions within the IE63 center region showed that S165, S173, and S185 were phosphorylated by cellular kinases. Four mutations that changed two putative nuclear localization signal (NLS) sequences altered IE63 distribution to a cytoplasmic/nuclear pattern. Only three of 22 mutations in ORF63 were compatible with recovery of infectious VZV from our cosmids, but infectivity was restored by inserting intact ORF63 into each mutated cosmid. The viable IE63 mutants had a single alanine substitution, altering T171, S181, or S185. These mutants, rOKA/ORF63rev[T171], rOKA/ORF63rev[S181], and rOKA/ORF63rev[S185], produced less infectious virus and had a decreased plaque phenotype in vitro. ORF47 kinase protein and glycoprotein E (gE) synthesis was reduced, indicating that IE63 contributed to optimal expression of early and late gene products. The three IE63 mutants replicated in skin xenografts in the SCIDhu mouse model, but virulence was markedly attenuated. In contrast, infectivity in T-cell xenografts was not altered. Comparative analysis suggested that IE63 resembled the herpes simplex virus type 1 U(S)1.5 protein, which is expressed colinearly with ICP22 (U(S)1). In summary, most mutations of ORF63 made with our VZV cosmid system were lethal for infectivity. The few IE63 changes that were tolerated resulted in VZV mutants with an impaired capacity to replicate in vitro. However, the IE63 mutants were attenuated in skin but not T cells in vivo, indicating that the contribution of the IE63 tegument/regulatory protein to VZV pathogenesis depends upon the differentiated human cell type which is targeted for infection within the intact tissue microenvironment.
Figures

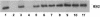



References
-
- Arvin, A. M. 2001. Varicella-zoster virus, p. 2731-2767. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott-Raven, Philadelphia, Pa.
-
- Besser, J., M. H. Sommer, L. Zerboni, C. Bagowski, H. Ito, J. Moffat, Ku, C.-C. and A. M. Arvin. 2003. Differentiation of varicella-zoster virus ORF47 protein kinase and IE62 protein binding domains and their contributions to replication in human skin xenografts in the SCIDhu mouse. J. Virol. 77:5964-5974. - PMC - PubMed
-
- Blom, N., S. Gammeltoft, und S. Brunak. 1999. Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J. Mol. Biol. 294:1351-1362. - PubMed
-
- Bontems, S., Valentin. D., E L. Baudox, B. Rentier, Sadzot-Delvaux, C., and J. Piette. 2002. Phosphorylation of varicella-zoster virus IE63 protein by casein kinases influences its cellular localization and gene regulation activity. J. Biol. Chem. 277:21050-21060. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

