Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes
- PMID: 14722292
- PMCID: PMC321368
- DOI: 10.1128/jvi.78.3.1375-1383.2004
Compensatory link between fusion and endocytosis of human immunodeficiency virus type 1 in human CD4 T lymphocytes
Abstract
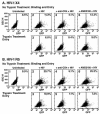
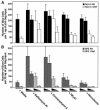
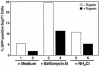
Virions of the type 1 human immunodeficiency virus (HIV-1) can enter target cells by fusion or endocytosis, with sharply different functional consequences. Fusion promotes productive infection of the target cell, while endocytosis generally leads to virion inactivation in acidified endosomes or degradation in lysosomes. Virion fusion and endocytosis occur equally in T cells, but these pathways have been regarded as independent because endocytosis of HIV virions requires neither CD4 nor CCR5/CXCR4 engagement in HeLa-CD4 cells. Using flow cytometric techniques to assess the binding and entry of green fluorescent protein (GFP)-Vpr-labeled HIV virions into primary peripheral blood mononuclear cells, we have found that HIV fusion and endocytosis are restricted to the CD4-expressing subset of cells and that both pathways commonly require the initial binding of HIV virions to surface CD4 receptors. Blockade of CXCR4-tropic HIV virion fusion with AMD3100, a CXCR4-specific entry inhibitor, increased virion entry via the endocytic pathway. Similarly, inhibition of endosome acidification with bafilomycin A1, concanamycin A, or NH(4)Cl enhanced entry via the fusion pathway. Although fusion remained dependent on CD4 and chemokine receptor binding, the endosome inhibitors did not alter surface expression of CD4 and CXCR4. These results suggest that fusion in the presence of the endosome inhibitors likely occurs within nonacidified endosomes. However, the ability of these inhibitors to impair vesicle trafficking from early to late endosomes in some cells could also increase the recycling of these virion-containing endosomes to the cell surface, where fusion occurs. In summary, our results reveal an unexpected, CD4-mediated reciprocal relationship between the pathways governing HIV virion fusion and endocytosis.
Figures


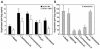
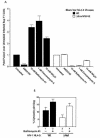
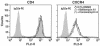
References
-
- Binley, J., and J. P. Moore. 1997. HIV-cell fusion. The viral mousetrap. Nature 387:346-348. - PubMed
-
- Bridger, G. J., R. T. Skerlj, D. Thornton, S. Padmanabhan, S. A. Martellucci, G. W. Henson, M. J. Abrams, N. Yamamoto, K. De Vreese, R. Pauwels, et al. 1995. Synthesis and structure-activity relationships of phenylenebis(methylene)-linked bis-tetraazamacrocycles that inhibit HIV replication. Effects of macrocyclic ring size and substituents on the aromatic linker. J. Med. Chem. 38:366-378. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

