Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival
- PMID: 14722611
- PMCID: PMC312596
- DOI: 10.1172/JCI19383
Soluble VEGF isoforms are essential for establishing epiphyseal vascularization and regulating chondrocyte development and survival
Abstract
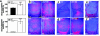
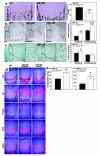
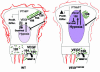
VEGF is crucial for metaphyseal bone vascularization. In contrast, the angiogenic factors required for vascularization of epiphyseal cartilage are unknown, although this represents a developmentally and clinically important aspect of bone growth. The VEGF gene is alternatively transcribed into VEGF(120), VEGF(164), and VEGF(188) isoforms that differ in matrix association and receptor binding. Their role in bone development was studied in mice expressing single isoforms. Here we report that expression of only VEGF(164) or only VEGF(188) (in VEGF(188/188) mice) was sufficient for metaphyseal development. VEGF(188/188) mice, however, showed dwarfism, disrupted development of growth plates and secondary ossification centers, and knee joint dysplasia. This phenotype was at least partly due to impaired vascularization surrounding the epiphysis, resulting in ectopically increased hypoxia and massive chondrocyte apoptosis in the interior of the epiphyseal cartilage. In addition to the vascular defect, we provide in vitro evidence that the VEGF(188) isoform alone is also insufficient to regulate chondrocyte proliferation and survival responses to hypoxia. Consistent herewith, chondrocytes in or close to the hypoxic zone in VEGF(188/188) mice showed increased proliferation and decreased differentiation. These findings indicate that the insoluble VEGF(188) isoform is insufficient for establishing epiphyseal vascularization and regulating cartilage development during endochondral bone formation.
Figures



References
-
- Pfander D, Cramer T, Schipani E, Johnson RS. HIF-1α controls extracellular matrix synthesis by epiphyseal chondrocytes. J. Cell Sci. 2003;116:1819–1826. - PubMed
-
- Karsenty G, Wagner EF. Reaching a genetic and molecular understanding of skeletal development. Dev. Cell. 2002;2:389–406. - PubMed
-
- Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000;6:389–395. - PubMed
-
- Robinson CJ, Stringer SE. The splice variants of vascular endothelial growth factor (VEGF) and their receptors. J. Cell Sci. 2001;114:853–865. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

