Bone marrow-derived progenitor cells in pulmonary fibrosis
- PMID: 14722616
- PMCID: PMC310750
- DOI: 10.1172/JCI18847
Bone marrow-derived progenitor cells in pulmonary fibrosis
Abstract
The origin of fibroblasts in pulmonary fibrosis is assumed to be intrapulmonary, but their extrapulmonary origin and especially derivation from bone marrow (BM) progenitor cells has not been ruled out. To examine this possibility directly, adult mice were durably engrafted with BM isolated from transgenic mice expressing enhanced GFP. Induction of pulmonary fibrosis in such chimera mice by endotracheal bleomycin (BLM) injection caused large numbers of GFP(+) cells to appear in active fibrotic lesions, while only a few GFP(+) cells could be identified in control lungs. Flow-cytometric analysis of lung cells confirmed the BLM-induced increase in GFP(+) cells in chimera mice and revealed a significant increase in GFP(+) cells that also express type I collagen. GFP(+) lung fibroblasts isolated from chimera mice expressed collagen and telomerase reverse transcriptase but not alpha-smooth muscle actin. Treatment of isolated GFP(+) fibroblasts with TGF-beta failed to induce myofibroblast differentiation. Cultured lung fibroblasts expressed the chemokine receptors CXCR4 and CCR7 and responded chemotactically to their cognate ligands, stromal cell-derived factor-1 alpha and secondary lymphoid chemokine, respectively. Thus the collagen-producing lung fibroblasts in pulmonary fibrosis can also be derived from BM progenitor cells.
Figures
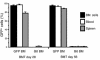
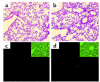


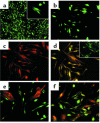

Comment in
-
The bone marrow leaves its scar: new concepts in pulmonary fibrosis.J Clin Invest. 2004 Jan;113(2):180-2. doi: 10.1172/JCI20782. J Clin Invest. 2004. PMID: 14722608 Free PMC article. Review.
References
-
- Blau HM, Brazelton TR, Weimann JM. The evolving concept of a stem cell: entity or function? Cell. 2001;105:829–841. - PubMed
-
- Ferrari G, et al. Muscle regeneration by bone marrow-derived myogenic progenitors. Science. 1998;279:1528–1530. - PubMed
-
- Orlic D, et al. Bone marrow cells regenerate infarcted myocardium. Nature. 2001;410:701–705. - PubMed
-
- Lagasse E, et al. Purified hematopoietic stem cells can differentiate into hepatocytes in vivo. Nat. Med. 2000;6:1229–1234. - PubMed
-
- Krause DS, et al. Multi-organ, multi-lineage engraftment by a single bone marrow-derived stem cell. Cell. 2001;105:369–377. - PubMed

