Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions
- PMID: 14722620
- PMCID: PMC310746
- DOI: 10.1172/JCI11548
Translational upregulation of folate receptors is mediated by homocysteine via RNA-heterogeneous nuclear ribonucleoprotein E1 interactions
Abstract
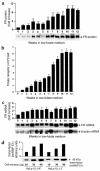
Cellular acquisition of folate is mediated by folate receptors (FRs) in many malignant and normal human cells. Although FRs are upregulated in folate deficiency and downregulated following folate repletion, the mechanistic basis for this relationship is unclear. Previously we demonstrated that interaction of an 18-base cis-element in the 5'-untranslated region of FR mRNA and a cystolic trans-factor (heterogeneous nuclear ribonucleoprotein E1 [hnRNP E1]) is critical for FR synthesis. However, the molecular mechanisms controlling this interaction, especially within the context of FR regulation and folate status, have remained obscure. Human cervical carcinoma cells exhibited progressively increasing upregulation of FRs after shifting of folate-replete cells to low-folate media, without a proportionate rise in FR mRNA or rise in hnRNP E1. Translational FR upregulation was accompanied by a progressive accumulation of the metabolite homocysteine within cultured cells, which stimulated interaction of the FR mRNA cis-element and hnRNP E1 as well as FR biosynthesis in a dose-dependent manner. Abrupt reversal of folate deficiency also led to a rapid parallel reduction in homocysteine and FR biosynthesis to levels observed in folate-replete cells. Collectively, these results suggest that homocysteine is the key modulator of translational upregulation of FRs and establishes the linkage between perturbed folate metabolism and coordinated upregulation of FRs.
Figures
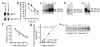
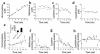
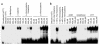



References
-
- Antony, A.C. 2000. Megaloblastic anemias. In Hematology: basic principles and practice. R. Hoffman et al., editors. Churchill-Livingstone. New York, New York, USA. 446–485.
-
- Antony AC. Folate receptors. Annu. Rev. Nutr. 1996;16:501–521. - PubMed
-
- Antony AC. The biological chemistry of folate receptors. Blood. 1992;79:2807–2820. - PubMed
-
- McHugh M, Cheng YC. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells. J. Biol. Chem. 1979;254:11312–11318. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

