Intrinsic cellular currents and the temporal precision of EPSP-action potential coupling in CA1 pyramidal cells
- PMID: 14724200
- PMCID: PMC1664854
- DOI: 10.1113/jphysiol.2003.052225
Intrinsic cellular currents and the temporal precision of EPSP-action potential coupling in CA1 pyramidal cells
Abstract
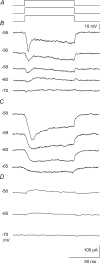
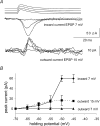
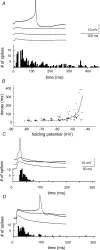
We examined relations between cellular currents activated near firing threshold and the initiation of action potentials by excitatory postsynaptic potentials (EPSPs) in CA1 pyramidal cells in vitro. Small voltage steps elicited sequences of inward-outward currents at hyperpolarized potentials, but evoked largely inward currents at near threshold potentials. Similarly small EPSP-like waveforms initiated largely inward currents while larger stimuli evoked sequences of inward followed by outward currents. Shorter rise times of EPSP-like waveforms accentuated a transient component of inward currents. Voltage clamp data were consistent with the voltage dependence of current clamp responses to injection of EPSP shaped waveforms. Small events were prolonged at subthreshold potentials and could elicit action potentials at long latencies while responses to larger EPSP waveforms showed less voltage dependence and tended to induce spikes at shorter, less variable latencies. The precision of action potentials initiated by white noise depended also on stimulus amplitude. High variance stimuli induced firing with high precision, while the timing of spikes induced by lower variance signals was more variable between trials. In voltage clamp records, high variance noise commands induced sequences of inward followed by outward currents, while lower variance versions of the same commands elicited purely inward currents. These data suggest that larger synaptic stimuli recruit outward as well as inward currents. The resulting inward-outward current sequences enhance the temporal precision of EPSP-spike coupling. Thus, CA1 pyramidal cells initiate action potentials with different temporal precision, depending on stimulus properties.
Figures


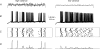

References
-
- Abbott LF, Nelson SB. Synaptic plasticity: taming the beast. Nat Neurosci. 2000;3(Suppl.):1178–1183. - PubMed
-
- Aksay E, Gamkrelidze G, Seung HS, Baker R, Tank DW. In vivo intracellular recording and perturbation of persistent activity in a neural integrator. Nat Neurosci. 2001;4:184–193. - PubMed
-
- Bolshakov VY, Siegelbaum SA. Regulation of hippocampal transmitter release during development and long-term potentiation. Science. 1995;269:1730–1734. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

