Dynamic properties of corticogeniculate excitatory transmission in the rat dorsal lateral geniculate nucleus in vitro
- PMID: 14724201
- PMCID: PMC1664892
- DOI: 10.1113/jphysiol.2003.052720
Dynamic properties of corticogeniculate excitatory transmission in the rat dorsal lateral geniculate nucleus in vitro
Abstract
The feedback excitation from the primary visual cortex to principal cells in the dorsal lateral geniculate nucleus (dLGN) is markedly enhanced with firing frequency. This property presumably reflects the ample short-term plasticity at the corticogeniculate synapse. The present study aims to explore corticogeniculate excitatory postsynaptic currents (EPSCs) evoked by brief trains of stimulation with whole-cell patch-clamp recordings in dLGN slices from DA-HAN rats. The EPSCs rapidly increased in amplitude with the first two or three impulses followed by a more gradual growth. A double exponential function with time constants 39 and 450 ms empirically described the growth for 5-25Hz trains. For lower train frequencies (down to 1Hz) a third component with time constant 4.8 s had to be included. The different time constants are suggested to represent fast and slow components of facilitation and augmentation. The time constant of the fast component changed with the extracellular calcium ion concentration as expected for a facilitation mechanism involving an endogenous calcium buffer that is more efficiently saturated with larger calcium influx. Concerning the function of the corticogeniculate feedback pathway, the different components of short-term plasticity interacted to increase EPSC amplitudes on a linear scale to firing frequency in the physiological range. This property makes the corticogeniculate synapse well suited to function as a neuronal amplifier that enhances the thalamic transfer of visual information to the cortex.
Figures
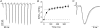
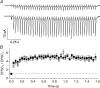

 ). Lines are least-sum-of-squares fits of a double exponential function as in Fig. 1 with the same time constants τ1 = 39 ms and τ2 = 450 ms. B, diagram comparing EPSC amplitudes at different points in time (t) for 5–25Hz trains. Data points are EPSC amplitude at, or immediately preceding t = 0.2 or 0.4 s. Continuous lines are linear regressions with Δyt = 0.2s= 0.23 and Δyt = 0.4s= 0.25. The dotted line is the theoretical Fss (×) regression line with Δyt =∞= 0.26 at the theoretical point in time t=∞. Other markers are the same as in A. Data points in A and B are average values ±
). Lines are least-sum-of-squares fits of a double exponential function as in Fig. 1 with the same time constants τ1 = 39 ms and τ2 = 450 ms. B, diagram comparing EPSC amplitudes at different points in time (t) for 5–25Hz trains. Data points are EPSC amplitude at, or immediately preceding t = 0.2 or 0.4 s. Continuous lines are linear regressions with Δyt = 0.2s= 0.23 and Δyt = 0.4s= 0.25. The dotted line is the theoretical Fss (×) regression line with Δyt =∞= 0.26 at the theoretical point in time t=∞. Other markers are the same as in A. Data points in A and B are average values ±
 ), 2 (
), 2 ( ) and 1Hz (
) and 1Hz ( ). Lines are exponential functions with time constants (τ1= 39 ms, τ2= 0.45 s and τ3= 4.8 s) transformed from the temporal domain to stimulus number (n). B, EPSCn/EPSC1 at 50 (□) and 25Hz (▪) over time. C, contribution of each exponential component in curve fits in A plotted against frequency (K1, ⋄; K2, ♦; K3,
). Lines are exponential functions with time constants (τ1= 39 ms, τ2= 0.45 s and τ3= 4.8 s) transformed from the temporal domain to stimulus number (n). B, EPSCn/EPSC1 at 50 (□) and 25Hz (▪) over time. C, contribution of each exponential component in curve fits in A plotted against frequency (K1, ⋄; K2, ♦; K3,  ). Continuous lines are curve fits; for K1 a linear regression between 2 and 25Hz (ΔyK1= 0.20 versus frequency), for K2 a linear regression for data between 5 and 25Hz (ΔyK2= 0.07) and for K3 an exponential decay. Dotted line is a regression line for K2+K3 (×; ΔyK2+K3= 0.06). Further details in the text. Average values ±
). Continuous lines are curve fits; for K1 a linear regression between 2 and 25Hz (ΔyK1= 0.20 versus frequency), for K2 a linear regression for data between 5 and 25Hz (ΔyK2= 0.07) and for K3 an exponential decay. Dotted line is a regression line for K2+K3 (×; ΔyK2+K3= 0.06). Further details in the text. Average values ±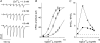
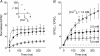
References
-
- Ahlsén G, Grant K, Lindström S. Monosynaptic excitation of principal cells in the lateral geniculate nucleus by corticofugal fibers. Brain Res. 1982;234:454–458. - PubMed
-
- Ahlsén G, Lindström S, Lo F-S. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Res. 1985;58:134–143. - PubMed
-
- Bartlett EL, Smith PH. Effects of paired-pulse and repetitive stimulation on neurons in the rat medial geniculate body. Neuroscience. 2002;113:957–974. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

