Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus
- PMID: 14724232
- PMCID: PMC6729997
- DOI: 10.1523/JNEUROSCI.3358-03.2004
Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus
Abstract
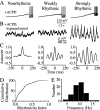
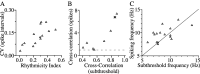
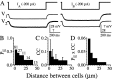
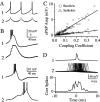
The inhibitory neurons of the thalamic reticular nucleus (TRN) contribute to the generation of widespread oscillations in the thalamocortical system. Some TRN neurons are interconnected by electrical synapses, and here we tested the possibility that electrical synapses mediate rhythmic synchrony in juvenile rats. Both the incidence and strength of electrical coupling between pairs of TRN neurons were a steep function of intersomatic distance, and coupling was absent at distances >40 microm. Presynaptic spike bursts evoked much larger electrical postsynaptic potentials than did single presynaptic spikes. Activation of metabotropic glutamate receptors (mGluRs) with a bath-applied agonist or an endogenous ligand released during tetanic stimulation induced robust rhythms of the subthreshold membrane potential, with a mean frequency of approximately 10 Hz. In the absence of fast chemical synaptic transmission, subthreshold rhythms and the action potentials that they evoked were well synchronized between closely spaced, electrically coupled pairs; rhythms in noncoupled cells were not synchronized. The results suggest that electrical synapses can coordinate spindle-frequency rhythms among small clusters of mGluR-activated TRN cells.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
