The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development
- PMID: 14724251
- PMCID: PMC6730001
- DOI: 10.1523/JNEUROSCI.3408-03.2004
The central fragment of Reelin, generated by proteolytic processing in vivo, is critical to its function during cortical plate development
Abstract
Reelin is a large extracellular protein that controls cortical development. It binds to lipoprotein receptors very-low-density lipoprotein receptor and apolipoprotein-E receptor type 2, thereby inducing phosphorylation of the adapter Dab1. In vivo, Reelin is cleaved into three fragments, but their respective function is unknown. Here we show the following: (1) the central fragment is necessary and sufficient for receptor binding in vitro and for Dab1 phosphorylation in neuronal cultures; (2) Reelin does not bind the protocadherin cadherin-related neuronal receptor (CNR1) as reported previously; (3) Reelin and its central fragment are equally able to rescue the reeler phenotype in a slice culture assay; and (4) anti-receptor antibodies can induce Dab1 phosphorylation but do not correct the reeler phenotype in slices. These observations show that the function of Reelin is critically dependent on the central fragment generated by processing but primarily independent of interactions with CNR1 and on the N-terminal region. They also indicate that events acting in parallel to Dab1 phosphorylation might be required for full activity.
Figures

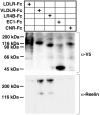


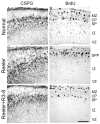

References
-
- Andersen OM, Benhayon D, Curran T, Willnow TE (2003) Differential binding of ligands to the apolipoprotein e receptor 2. Biochemistry 42: 9355-9364. - PubMed
-
- Arnaud L, Ballif BA, Forster E, Cooper JA (2003) Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol 13: 9-17. - PubMed
-
- Bar I, Tissir F, Lambert De Rouvroit C, De Backer O, Goffinet AM (2003) The gene encoding disabled-1 (DAB1), the intracellular adaptor of the Reelin pathway, reveals unusual complexity in human and mouse. J Biol Chem 278: 5802-5812. - PubMed
-
- Bock HH, Herz J (2003) Reelin activates SRC family tyrosine kinases in neurons. Curr Biol 13: 18-26. - PubMed
-
- Boucher P, Liu P, Gotthardt M, Hiesberger T, Anderson RG, Herz J (2002) Platelet-derived growth factor mediates tyrosine phosphorylation of the cytoplasmic domain of the low density lipoprotein receptor-related protein in caveolae. J Biol Chem 277: 15507-15513. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
