Pharmacological enhancement of beta-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff Patients
- PMID: 14724290
- PMCID: PMC2904802
- DOI: 10.1074/jbc.M308523200
Pharmacological enhancement of beta-hexosaminidase activity in fibroblasts from adult Tay-Sachs and Sandhoff Patients
Abstract
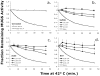
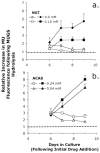
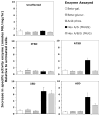
Tay-Sachs and Sandhoff diseases are lysosomal storage disorders that result from an inherited deficiency of beta-hexosaminidase A (alphabeta). Whereas the acute forms are associated with a total absence of hexosaminidase A and early death, the chronic adult forms exist with activity and protein levels of approximately 5%, and unaffected individuals have been found with only 10% of normal levels. Surprisingly, almost all disease-associated missense mutations do not affect the active site of the enzyme but, rather, inhibit its ability to obtain and/or retain its native fold in the endoplasmic reticulum, resulting in its retention and accelerated degradation. By growing adult Tay-Sachs fibroblasts in culture medium containing known inhibitors of hexosaminidase we have raised the residual protein and activity levels of intralysosomal hexosaminidase A well above the critical 10% of normal levels. A similar effect was observed in fibroblasts from an adult Sandhoff patient. We propose that these hexosaminidase inhibitors function as pharmacological chaperones, enhancing the stability of the native conformation of the enzyme, increasing the amount of hexosaminidase A capable of exiting the endoplasmic reticulum for transport to the lysosome. Therefore, pharmacological chaperones could provide a novel approach to the treatment of adult Tay-Sachs and possibly Sandhoff diseases.
Figures





References
-
- Gravel RA, Clarke JTR, Kaback MM, Mahuran D, Sandhoff K, Suzuki K. In: The Metabolic and Molecular Bases of Inherited Disease. 7. Scriver CR, Beaudet AL, Sly WS, Valle D, editors. Vol. 2. McGraw-Hill; New York: 1995. pp. 2839–2879.
-
- Hou Y, Tse R, Mahuran DJ. Biochemistry. 1996;35:3963–3969. - PubMed
-
- Mahuran DJ. Biochim Biophys Acta. 1991;1096:87–94. - PubMed
-
- Dlott B, D’Azzo A, Quon DVK, Neufeld EF. J Biol Chem. 1990;265:17921–17927. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

