Antimicrobial treatment guidelines for acute bacterial rhinosinusitis
- PMID: 14726904
- PMCID: PMC7118847
- DOI: 10.1016/j.otohns.2003.12.003
Antimicrobial treatment guidelines for acute bacterial rhinosinusitis
Erratum in
- Otolaryngol Head Neck Surg. 2004 Jun;130(6):794-6
Abstract
Treatment guidelines developed by the Sinus and Allergy Health Partnership for acute bacterial rhinosinusitis (ABRS) were originally published in 2000. These guidelines were designed to: (1) educate clinicians and patients (or patients’ families) about the differences between viral and bacterial rhinosinusitis; (2) reduce the use of antibiotics for nonbacterial nasal/sinus disease; (3) provide recommendations for the diagnosis and optimal treatment of ABRS; (4) promote the use of appropriate antibiotic therapy when bacterial infection is likely; and (5) describe the current understanding of pharmacokinetic and pharmacodynamics and how they relate to the effectiveness of antimicrobial therapy. The original guidelines are updated here to include the most recent information on management principles, antimicrobial susceptibility patterns, and therapeutic options.
Burden of disease: An estimated 20 million cases of ABRS occur annually in the United States. According to National Ambulatory Medical Care Survey (NAMCS) data, sinusitis is the fifth most common diagnosis for which an antibiotic is prescribed. Sinusitis accounted for 9% and 21% of all pediatric and adult antibiotic prescriptions, respectively, written in 2002. The primary diagnosis of sinusitis results in expenditures of approximately $3.5 billion per year in the United States.
Definition and diagnosis of ABRS: ABRS is most often preceded by a viral upper respiratory tract infection (URI). Allergy, trauma, dental infection, or other factors that lead to inflammation of the nose and paranasal sinuses may also predispose individuals to developing ABRS.
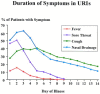
Patients with a “common cold” (viral URI) usually report some combination of the following symptoms: sneezing, rhinorrhea, nasal congestion, hyposmia/anosmia, facial pressure, postnasal drip, sore throat, cough, ear fullness, fever, and myalgia. A change in the color or the characteristic of the nasal discharge is not a specific sign of a bacterial infection. Bacterial superinfection may occur at any time during the course of a viral URI. The risk that bacterial superinfection has occurred is greater if the illness is still present after 10 days. Because there may be cases that fall out of the “norm” of this typical progression, practicing clinicians need to rely on their clinical judgment when using these guidelines. In general, however, a diagnosis of ABRS may be made in adults or children with symptoms of a viral URI that have not improved after 10 days or worsen after 5 to 7 days. There may be some or all of the following signs and symptoms: nasal drainage, nasal congestion, facial pressure/pain (especially when unilateral and focused in the region of a particular sinus), postnasal drainage, hyposmia/anosmia, fever, cough, fatigue, maxillary dental pain, and ear pressure/fullness.
Physical examination provides limited information in the diagnosis of ABRS.
While sometimes helpful, plain film radiographs, computed tomography (CT), and magnetic resonance imaging scans are not necessary for cases of ABRS.
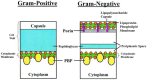
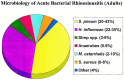
Microbiology of ABRS: The most common bacterial species isolated from the maxillary sinuses of patients with ABRS are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis, the latter being more common in children. Other streptococcal species, anaerobic bacteria and Staphylococcus aureus cause a small percentage of cases.
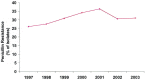
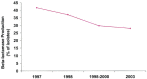
Bacterial resistance in ABRS: The increasing prevalence of penicillin nonsusceptibility and resistance to other drug classes among S pneumoniae has been a problem in the United States, with 15% being penicillin-intermediate and 25% being penicillin-resistant in recent studies. Resistance to macrolides and trimethoprim/sulfamethoxazole (TMP/SMX) is also common in S pneumoniae. The prevalence of β-lactamase-producing isolates of H influenzae is approximately 30%, while essentially all M catarrhalis isolates produce β-lactamases. Resistance of H influenzae to TMP/SMX is also common.
Antimicrobial treatment guidelines for ABRS: These guidelines apply to both adults and children. When selecting antibiotic therapy for ABRS, the clinician should consider the severity of the disease, the rate of progression of the disease, and recent antibiotic exposure. The guidelines now divide patients with ABRS into two general categories: (1) those with mild symptoms who have not received antibiotics within the past 4 to 6 weeks, and (2) those with mild disease who have received antibiotics within the past 4 to 6 weeks or those with moderate disease regardless of recent antibiotic exposure. The difference in severity of disease does not imply infection with a resistant pathogen. Rather, this terminology indicates the relative degree of acceptance of possible treatment failure and the likelihood of spontaneous resolution of symptoms—patients with more severe symptoms are less likely to resolve their disease spontaneously. The primary goal of antibiotic therapy is to eradicate bacteria from the site of infection, which, in turn, helps (1) return the sinuses back to health; (2) decrease the duration of symptoms to allow patients to resume daily activities more quickly; (3) prevent severe complications such as meningitis and brain abscess; and (4) decrease the development of chronic disease. Severe or life-threatening infections with or without complications are rare, and are not addressed in these guidelines.
Prior antibiotic use is a major risk factor associated with the development of infection with antimicrobial-resistant strains. Because recent antimicrobial exposure increases the risk of carriage of and infection due to resistant organisms, antimicrobial therapy should be based upon the patient’s history of recent antibiotic use. The panel’s guidelines, therefore, stratify patients according to antibiotic exposure in the previous 4 to 6 weeks.
Lack of response to therapy at ≥72 hours is an arbitrary time established to define treatment failures. Clinicians should monitor the response to antibiotic therapy, which may include instructing the patient to call the office or clinic if symptoms persist or worsen over the next few days.
The predicted bacteriologic and clinical efficacy of antibiotics in adults and children has been determined according to mathematical modeling of ABRS developed by Michael Poole, MD, PhD, based on pathogen distribution, resolution rates without treatment, and in vitro microbiologic activity.
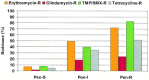
Antibiotics can be placed into the following relative rank order of predicted clinical efficacy for adults: 90% to 92% = respiratory fluoroquinolones (gatifloxacin, levofloxacin, moxifloxacin), ceftriaxone, high-dose amoxicillin/clavulanate (4 g/250 mg/day), and amoxicillin/clavulanate (1.75 g/250 mg/day); 83% to 88% = high-dose amoxicillin (4 g/day), amoxicillin (1.5 g/day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage), cefuroxime axetil, cefdinir, and TMP/SMX; 77% to 81% = doxycycline, clindamycin (based on gram-positive coverage only), azithromycin, clarithromycin and erythromycin, and telithromycin; 65% to 66% = cefaclor and loracarbef. The predicted spontaneous resolution rate in patients with a clinical diagnosis of ABRS is 62%.
Antibiotics can be placed into the following relative rank order of predicted clinical efficacy in children with ABRS: 91% to 92% = ceftriaxone, high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day) and amoxicillin/clavulanate (45 mg/6.4 mg per kg per day); 82% to 87% = high-dose amoxicillin (90 mg/kg per day), amoxicillin (45 mg/kg per day), cefpodoxime proxetil, cefixime (based on H influenzae and M catarrhalis coverage only), cefuroxime axetil, cefdinir, and TMP/SMX; and 78% to 80% = clindamycin (based on gram-positive coverage only), cefprozil, azithromycin, clarithromycin, and erythromycin; 67% to 68% = cefaclor and loracarbef. The predicted spontaneous resolution rate in untreated children with a presumed diagnosis of ABRS is 63%.
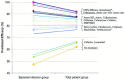
Recommendations for initial therapy for adult patients with mild disease (who have not received antibiotics in the previous 4 to 6 weeks) include the following choices: amoxicillin/clavulanate (1.75 to 4 g/250 mg per day), amoxicillin (1.5 to 4 g/day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. While TMP/SMX, doxycycline, azithromycin, clarithromycin, erythromycin, or telithromycin may be considered for patients with β-lactam allergies, bacteriologic failure rates of 20% to 25% are possible. Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4).When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
Recommendations for initial therapy for adults with mild disease who have received antibiotics in the previous 4 to 6 weeks or adults with moderate disease include the following choices: respiratory fluoroquinolone (eg, gatifloxacin, levofloxacin, moxifloxacin) or high-dose amoxicillin/clavulanate (4 g/250 mg per day). The widespread use of respiratory fluoroquinolones for patients with milder disease may promote resistance of a wide spectrum of organisms to this class of agents. Ceftriaxone (parenteral, 1 to 2 g/day for 5 days) or combination therapy with adequate gram-positive and negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. While the clinical effectiveness of ceftriaxone and these combinations for ABRS is unproven; the panel considers these reasonable therapeutic options based on the spectrum of activity of these agents and on data extrapolated from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days, as resistance quickly develops to this agent. Rifampin is also a well-known inducer of several cytochrome p450 isoenzymes and therefore has a high potential for drug interactions. Failure of a patient to respond to antimicrobial therapy after 72 hours of therapy should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 4). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent. Patients who have received effective antibiotic therapy and continue to be symptomatic may need further evaluation. A CT scan, fiberoptic endoscopy or sinus aspiration and culture may be necessary.
Recommendations for initial therapy for children with mild disease and who have not received antibiotics in the previous 4 to 6 weeks include the following: high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day), amoxicillin (90 mg/kg per day), cefpodoxime proxetil, cefuroxime axetil, or cefdinir. TMP/SMX, azithromycin, clarithromycin, or erythromycin is recommended if the patient has a history of immediate Type I hypersensitivity reaction to β-lactams. These antibiotics have limited effectiveness against the major pathogens of ABRS and bacterial failure of 20% to 25% is possible. The clinician should differentiate an immediate hypersensitivity reaction from other less dangerous side effects. Children with immediate hypersensitivity reactions to β-lactams may need: desensitization, sinus cultures, or other ancillary procedures and studies. Children with other types of reactions and side effects may tolerate one specific β-lactam, but not another. Failure to respond to antimicrobial therapy after 72 hours should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 5).When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent.
The recommended initial therapy for children with mild disease who have received antibiotics in the previous 4 to 6 weeks or children with moderate disease is high-dose amoxicillin/clavulanate (90 mg/6.4 mg per kg per day). Cefpodoxime proxetil, cefuroxime axetil, or cefdinir may be used if there is a penicillin allergy (eg, penicillin rash); in such instances, cefdinir is preferred because of high patient acceptance. TMP/SMX, azithromycin, clarithromycin, or erythromycin is recommended if the patient is β-lactam allergic, but these do not provide optimal coverage. Clindamycin is appropriate if S pneumoniae is identified as a pathogen. Ceftriaxone (parenteral, 50 mg/kg per day for 5 days) or combination therapy with adequate gram-positive and -negative coverage may also be considered. Examples of appropriate regimens of combination therapy include high-dose amoxicillin or clindamycin plus cefixime, or high-dose amoxicillin or clindamycin plus rifampin. The clinical effectiveness of ceftriaxone and these combinations for ABRS is unproven; the panel considers these reasonable therapeutic options based on spectrum of activity and on data extrapolated from acute otitis media studies. Rifampin should not be used as monotherapy, casually, or for longer than 10 to 14 days as resistance quickly develops to this agent. Failure to respond to antimicrobial therapy after 72 hours of therapy should prompt either a switch to alternate antimicrobial therapy or reevaluation of the patient (see Table 5). When a change in antibiotic therapy is made, the clinician should consider the limitations in coverage of the initial agent. Patients who have received effective antibiotic therapy and continue to be symptomatic may need further evaluation. A CT scan, fiberoptic endoscopy or sinus aspiration and culture may be necessary.
Figures







References
-
- Sinus and Allergy Health Partnership Antimicrobial treatment guidelines for acute bacterial Rhinosinusitis. Otolaryngol Head Neck Surg. 2000;123:S1–31. - PubMed
-
- Dowell S.F., Butler J.C., Giebink G.S. Acute otitis media: management and surveillance in an era of pneumococcal resistance—a report from the Drug-resistant Streptococcus pneumoniae Therapeutic Working Group. Pediatr Infect Dis J. 1999;18:1–9. - PubMed
-
- Gwaltney J.M., Jr, Phillips C.D., Miller R.D. Computed tomographic study of the common cold. N Engl J Med. 1994;330:25–30. - PubMed
-
- Berg O., Carenfelt C., Rystedt G. Occurrence of asymptomatic sinusitis in common cold and other acute ENT-infections. Rhinology. 1986;24:223–225. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

