Characterization of two types of ribosomal gene transcription in Xenopus laevis oocytes
- PMID: 1472871
- PMCID: PMC6057366
Characterization of two types of ribosomal gene transcription in Xenopus laevis oocytes
Abstract
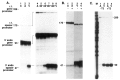
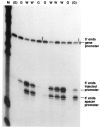
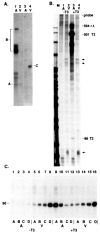
When the germinal vesicle of Xenopus laevis oocytes is translocated into the vegetal hemisphere by centrifugation, the normally silent ribosomal spacer promoters are strongly induced. This induction correlates with the permeability of the nuclear envelope to dextran of molecular weight 70 kDa, thus raising the possibility that the transcriptional changes are due to mixing of nuclear and cytoplasmic components. This basic observation prompted a thorough investigation of ribosomal gene transcription in centrifuged oocytes which had the germinal vesicle either in the animal half (A-oocytes) or in the vegetal half (V-oocytes). Two types of ribosomal gene transcription were characterized: (1) in A-oocytes, spacer promoters remain silent, transcription initiation is dependent on the upstream terminator T3, and transcription is highly processive and recognizes sites of RNA 3' end formation (like T2 and T3); (2) in V-oocytes, spacer promoters are induced, transcription initiation is independent of T3, but most transcripts terminate prematurely after less than 150 nt. Furthermore, the transcription machinery in V-oocytes does not respond to T2 or T3 signals. The implications of the present observations for our understanding of the regulation of the spacer promoters and of the function of the upstream terminator T3 are discussed.
Figures




References
-
- Bateman E. and Paule M. (1988), Cell 54, 985–992. - PubMed
-
- Boseley P., Moss T., Maechler M., Portmann R., and Birnstiel M. (1979), Cell 17, 19–31. - PubMed
-
- Botchan P., Reeder R. H., and Dawid I. B. (1977), Cell 11, 599–607. - PubMed
-
- De Winter R. F. J. and Moss T. (1986), Cell 44, 313–318. - PubMed
-
- Dingwall C. and Laskey R. A. (1986), Annu Rev Cell Biol 2, 367–390. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
