The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export
- PMID: 14729688
- PMCID: PMC321503
- DOI: 10.1128/JB.186.3.638-645.2004
The Streptococcus gordonii platelet binding protein GspB undergoes glycosylation independently of export
Abstract
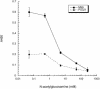
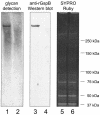
The binding of bacteria and platelets may play a central role in the pathogenesis of infective endocarditis. Platelet binding by Streptococcus gordonii strain M99 is predominantly mediated by the 286-kDa cell wall-anchored protein GspB. This unusually large protein lacks a typical amino-terminal signal peptide and is translocated from the cytoplasm via a dedicated transport system. A 14-kb segment just downstream of gspB encodes SecA2 and SecY2, two components of the GspB-specific transport system. The downstream segment also encodes several putative glycosyl transferases that may be responsible for the posttranslational modification of GspB. In this study, we compared the abilities of M99 and two GspB(-) mutant strains to bind various lectins. GspB was found to have affinity for lectins that bind N-acetylglucosamine. We also examined variant forms of GspB that lack a carboxy-terminal cell wall-anchoring domain and thus are free of covalent linkage to cell wall peptidoglycan. Like native GspB, these truncated proteins appear to be heavily glycosylated, as evidenced by migration during sodium dodecyl sulfate-polyacrylamide gel electrophoresis with an apparent molecular mass >100 kDa in excess of the predicted mass, negligible staining with conventional protein stains, and reactivity with hydrazide following periodate oxidation. Furthermore, analysis of the carbohydrate associated with the GspB variants by high-pH anion-exchange chromatography revealed the presence of approximately 70 to 100 monosaccharide residues per GspB polypeptide (primarily N-acetylglucosamine and glucose). Analysis of GspB in protoplasts of secA2 or secY2 mutant strains, which do not export GspB, indicates that GspB is glycosylated in the cytoplasm of these strains. The combined data suggest that the native GspB is a glycoprotein and that it may be glycosylated prior to export.
Figures

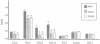



References
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1997. Current protocols in molecular biology. John Wiley & Sons, New York, N.Y.
-
- Bensing, B. A., and P. M. Sullam. 2002. An accessory sec locus of Streptococcus gordonii is required for export of the surface protein GspB and for normal levels of binding to human platelets. Mol. Microbiol. 44:1081-1094. - PubMed
-
- Benz, I., and M. A. Schmidt. 2002. Never say never again: protein glycosylation in pathogenic bacteria. Mol. Microbiol. 45:267-276. - PubMed
-
- Claverys, J. P., A. Dintilhac, E. V. Pestova, B. Martin, and D. A. Morrison. 1995. Construction and evaluation of new drug-resistance cassettes for gene disruption mutagenesis in Streptococcus pneumoniae, using an ami test platform. Gene 164:123-128. - PubMed
-
- Durack, D. T. 1975. Experimental bacterial endocarditis. IV. Structure and evolution of very early lesions. J. Pathol. 115:81-89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

