The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance
- PMID: 14729910
- PMCID: PMC341917
- DOI: 10.1105/tpc.016907
The Arabidopsis thaliana dihydroxyacetone phosphate reductase gene SUPPRESSSOR OF FATTY ACID DESATURASE DEFICIENCY1 is required for glycerolipid metabolism and for the activation of systemic acquired resistance
Abstract
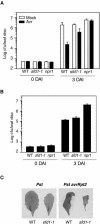
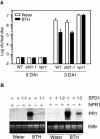
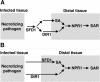
Systemic acquired resistance (SAR) is a broad-spectrum resistance mechanism in plants that is activated in naive organs after exposure of another organ to a necrotizing pathogen. The organs manifesting SAR exhibit an increase in levels of salicylic acid (SA) and expression of the PATHOGENESIS-RELATED1 (PR1) gene. SA signaling is required for the manifestation of SAR. We demonstrate here that the Arabidopsis thaliana suppressor of fatty acid desaturase deficiency1 (sfd1) mutation compromises the SAR-conferred enhanced resistance to Pseudomonas syringae pv maculicola. In addition, the sfd1 mutation diminished the SAR-associated accumulation of elevated levels of SA and PR1 gene transcript in the distal leaves of plants previously exposed to an avirulent pathogen. However, the basal resistance to virulent and avirulent strains of P. syringae and the accumulation of elevated levels of SA and PR1 gene transcript in the pathogen-inoculated leaves of sfd1 were not compromised. Furthermore, the application of the SA functional analog benzothiadiazole enhanced disease resistance in the sfd1 mutant plants. SFD1 encodes a putative dihydroxyacetone phosphate (DHAP) reductase, which complemented the glycerol-3-phosphate auxotrophy of the DHAP reductase-deficient Escherichia coli gpsA mutant. Plastid glycerolipid composition was altered in the sfd1 mutant plant, suggesting that SFD1 is involved in lipid metabolism and that an SFD1 product lipid(s) is important for the activation of SAR.
Figures





References
-
- Anderson, M.D., Chen, Z., and Klessig, D.F. (1998). Possible involvement of lipid peroxidation in salicylic acid-mediated induction of PR1 gene expression. Phytochemistry 47, 555–566.
-
- Bell, C.J., and Ecker, J.R. (1994). Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19, 137–144. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

