RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation
- PMID: 14729943
- PMCID: PMC321458
- DOI: 10.1128/MCB.24.3.945-953.2004
RNase MRP cleaves the CLB2 mRNA to promote cell cycle progression: novel method of mRNA degradation
Abstract
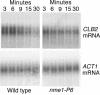
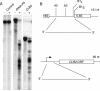
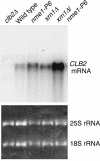
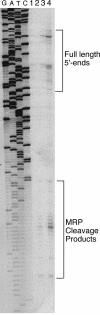
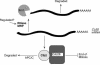
RNase mitochondrial RNA processing (RNase MRP) mutants have been shown to have an exit-from-mitosis defect that is caused by an increase in CLB2 mRNA levels, leading to increased Clb2p (B-cyclin) levels and a resulting late anaphase delay. Here we describe the molecular defect behind this delay. CLB2 mRNA normally disappears rapidly as cells complete mitosis, but the level remains high in RNase MRP mutants. This is in direct contrast to other exit-from-mitosis mutants and is the result of an increase in CLB2 mRNA stability. We found that highly purified RNase MRP cleaved the 5' untranslated region (UTR) of the CLB2 mRNA in several places in an in vitro assay. In vivo, we identified RNase MRP-dependent cleavage products on the CLB2 mRNA that closely matched in vitro products. Disposal of these products was dependent on the 5'-->3' exoribonuclease Xrn1 and not the exosome. Our results demonstrate that the endoribonuclease RNase MRP specifically cleaves the CLB2 mRNA in its 5'-UTR to allow rapid 5' to 3' degradation by the Xrn1 nuclease. Degradation of the CLB2 mRNA by the RNase MRP endonuclease provides a novel way to regulate the cell cycle that complements the protein degradation machinery. In addition, these results denote a new mechanism of mRNA degradation not seen before in the yeast Saccharomyces cerevisiae.
Figures



References
-
- Aulds, J., T. Cai, and M. E. Schmitt. 2002. RNase MRP from yeast to humans, cell cycle control and cartilage hair hypoplasia. Recent Res. Dev. Mol. Cell. Biol. 3:371-378.
-
- Beelman, C. A., and R. Parker. 1995. Degradation of mRNA in eukaryotes. Cell 81:179-183. - PubMed
-
- Cai, T., and M. E. Schmitt. 2001. Characterization of ribonuclease MRP function. Methods Enzymol. 342:135-142. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
