Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice
- PMID: 14729956
- PMCID: PMC321423
- DOI: 10.1128/MCB.24.3.1096-1105.2004
Evidence for a conserved function in synapse formation reveals Phr1 as a candidate gene for respiratory failure in newborn mice
Abstract
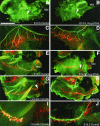
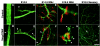
Genetic studies using a set of overlapping deletions centered at the piebald locus on distal mouse chromosome 14 have defined a genomic region associated with respiratory distress and lethality at birth. We have isolated and characterized the candidate gene Phr1 that is located within the respiratory distress critical genomic interval. Phr1 is the ortholog of the human Protein Associated with Myc as well as Drosophila highwire and Caenorhabditis elegans regulator of presynaptic morphology 1. Phr1 is expressed in the embryonic and postnatal nervous system. In mice lacking Phr1, the phrenic nerve failed to completely innervate the diaphragm. In addition, nerve terminal morphology was severely disrupted, comparable with the synaptic defects seen in the Drosophila hiw and C. elegans rpm-1 mutants. Although intercostal muscles were completely innervated, they also showed dysmorphic nerve terminals. In addition, sensory neuron terminals in the diaphragm were abnormal. The neuromuscular junctions showed excessive sprouting of nerve terminals, consistent with inadequate presynaptic stimulation of the muscle. On the basis of the abnormal neuronal morphology seen in mice, Drosophila, and C. elegans, we propose that Phr1 plays a conserved role in synaptic development and is a candidate gene for respiratory distress and ventilatory disorders that arise from defective neuronal control of breathing.
Figures




References
-
- Burgess, R. W., Q. T. Nguyen, Y. J. Son, J. W. Lichtman, and J. R. Sanes. 1999. Alternatively spliced isoforms of nerve- and muscle-derived agrin: their roles at the neuromuscular junction. Neuron 23:33-44. - PubMed
-
- Champliaud, M. F., H. P. Baden, M. Koch, W. Jin, R. E. Burgeson, and A. Viel. 2000. Gene characterization of sciellin (SCEL) and protein localization in vertebrate epithelia displaying barrier properties. Genomics 70:264-268. - PubMed
-
- Cole, F. S., A. Hamvas, and L. M. Nogee. 2001. Genetic disorders of neonatal respiratory function. Pediatr. Res. 50:157-162. - PubMed
-
- DeChiara, T. M., D. C. Bowen, D. M. Valenzuela, M. V. Simmons, W. T. Poueymirou, S. Thomas, E. Kinetz, D. L. Compton, E. Rojas, J. S. Park, C. Smith, P. S. DiStefano, D. J. Glass, S. J. Burden, and G. D. Yancopoulos. 1996. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell 85:501-512. - PubMed
-
- DiAntonio, A., A. P. Haghighi, S. L. Portman, J. D. Lee, A. M. Amaranto, and C. S. Goodman. 2001. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 412:449-452. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
