ERF nuclear shuttling, a continuous monitor of Erk activity that links it to cell cycle progression
- PMID: 14729966
- PMCID: PMC321421
- DOI: 10.1128/MCB.24.3.1206-1218.2004
ERF nuclear shuttling, a continuous monitor of Erk activity that links it to cell cycle progression
Abstract
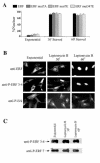
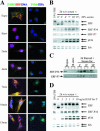
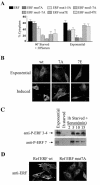
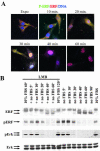
The ets domain transcriptional repressor ERF is an effector of the receptor tyrosine kinase/Ras/Erk pathway, which, it has been suggested, is regulated by subcellular localization as a result of Erk-dependent phosphorylation and is capable of suppressing cell proliferation and ras-induced tumorigenicity. Here, we analyze the effect of ERF phosphorylation on nuclear import and export, the timing of its phosphorylation and dephosphorylation in relation to its subcellular location, Erk activity, and the requirements for ERF-induced cell cycle arrest. Our findings indicate that ERF continuously shuttles between the nucleus and the cytoplasm and that both phosphorylation and dephosphorylation of ERF occur within the nucleus. While nuclear import is not affected by phosphorylation, ERF nuclear export and cytoplasmic release require multisite phosphorylation and dephosphorylation. ERF export is CRM1 dependent, although ERF does not have a detectable nuclear export signal. ERF phosphorylation and export correlate with the levels of nuclear Erk activity. The cell cycle arrest induced by nonphosphorylated ERF requires the wild-type retinoblastoma protein and can be suppressed by overexpression of cyclin. These data suggest that ERF may be a very sensitive and constant sensor of Erk activity that can affect cell cycle progression through G(1), providing another link between the Ras/Erk pathway and cellular proliferation.
Figures



References
-
- Alt, J. R., A. B. Gladden, and J. A. Diehl. 2002. p21(Cip1) promotes cyclin D1 nuclear accumulation via direct inhibition of nuclear export. J. Biol. Chem. 277:8517-8523. - PubMed
-
- Argentini, M., N. Barboule, and B. Wasylyk. 2001. The contribution of the acidic domain of MDM2 to p53 and MDM2 stability. Oncogene 20:1267-1275. - PubMed
-
- Athanasiou, M., L. LeGallic, D. K. Watson, D. G. Blair, and G. Mavrothalassitis. 2000. Suppression of the Ewing's sarcoma phenotype by FLI1/ERF repressor hybrids. Cancer Gene Ther. 7:1188-1195. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
