The ctf13-30/CTF13 genomic haploinsufficiency modifier screen identifies the yeast chromatin remodeling complex RSC, which is required for the establishment of sister chromatid cohesion
- PMID: 14729968
- PMCID: PMC321452
- DOI: 10.1128/MCB.24.3.1232-1244.2003
The ctf13-30/CTF13 genomic haploinsufficiency modifier screen identifies the yeast chromatin remodeling complex RSC, which is required for the establishment of sister chromatid cohesion
Abstract
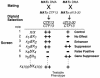
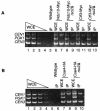
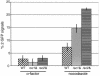
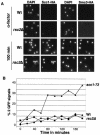
The budding yeast centromere-kinetochore complex ensures high-fidelity chromosome segregation in mitosis and meiosis by mediating the attachment and movement of chromosomes along spindle microtubules. To identify new genes and pathways whose function impinges on chromosome transmission, we developed a genomic haploinsufficiency modifier screen and used ctf13-30, encoding a mutant core kinetochore protein, as the reference point. We demonstrate through a series of secondary screens that the genomic modifier screen is a successful method for identifying genes that encode nonessential proteins required for the fidelity of chromosome segregation. One gene isolated in our screen was RSC2, a nonessential subunit of the RSC chromatin remodeling complex. rsc2 mutants have defects in both chromosome segregation and cohesion, but the localization of kinetochore proteins to centromeres is not affected. We determined that, in the absence of RSC2, cohesin could still associate with chromosomes but fails to achieve proper cohesion between sister chromatids, indicating that RSC has a role in the establishment of cohesion. In addition, numerous subunits of RSC were affinity purified and a new component of RSC, Rtt102, was identified. Our work indicates that only a subset of the nonessential RSC subunits function in maintaining chromosome transmission fidelity.
Figures

References
-
- Angus-Hill, M. L., A. Schlichter, D. Roberts, H. Erdjument-Bromage, P. Tempst, and B. R. Cairns. 2001. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell 7:741-751. - PubMed
-
- Bennett, C. B., L. K. Lewis, G. Karthikeyan, K. S. Lobachev, Y. H. Jin, J. F. Sterling, J. R. Snipe, and M. A. Resnick. 2001. Genes required for ionizing radiation resistance in yeast. Nat. Genet. 29:426-434. - PubMed
-
- Biggins, S., and C. E. Walczak. 2003. Captivating capture: how microtubules attach to kinetochores. Curr. Biol. 13:R449-R460. - PubMed
-
- Bloom, K. S., and J. Carbon. 1982. Yeast centromere DNA is in a unique and highly ordered structure in chromosomes and small circular minichromosomes. Cell 29:305-317. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
