Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity
- PMID: 14729974
- PMCID: PMC321434
- DOI: 10.1128/MCB.24.3.1301-1312.2004
Nicotinamide clearance by Pnc1 directly regulates Sir2-mediated silencing and longevity
Abstract
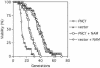
The Saccharomyces cerevisiae Sir2 protein is an NAD(+)-dependent histone deacetylase (HDAC) that functions in transcriptional silencing and longevity. The NAD(+) salvage pathway protein, Npt1, regulates Sir2-mediated processes by maintaining a sufficiently high intracellular NAD(+) concentration. However, another NAD(+) salvage pathway component, Pnc1, modulates silencing independently of the NAD(+) concentration. Nicotinamide (NAM) is a by-product of the Sir2 deacetylase reaction and is a natural Sir2 inhibitor. Pnc1 is a nicotinamidase that converts NAM to nicotinic acid. Here we show that recombinant Pnc1 stimulates Sir2 HDAC activity in vitro by preventing the accumulation of NAM produced by Sir2. In vivo, telomeric, rDNA, and HM silencing are differentially sensitive to inhibition by NAM. Furthermore, PNC1 overexpression suppresses the inhibitory effect of exogenously added NAM on silencing, life span, and Hst1-mediated transcriptional repression. Finally, we show that stress suppresses the inhibitory effect of NAM through the induction of PNC1 expression. Pnc1, therefore, positively regulates Sir2-mediated silencing and longevity by preventing the accumulation of intracellular NAM during times of stress.
Figures






References
-
- Anderson, R. M., K. J. Bitterman, J. G. Wood, O. Medvedik, H. Cohen, S. S. Lin, J. K. Manchester, J. I. Gordon, and D. A. Sinclair. 2002. Manipulation of a nuclear NAD+ salvage pathway delays aging without altering steady-state NAD+ levels. J. Biol. Chem. 277:18881-18890. - PubMed
-
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 2000. Current protocols in molecular biology, vol. 2. John Wiley & Sons, Inc., New York, N.Y.
-
- Bitterman, K. J., R. M. Anderson, H. Y. Cohen, M. Latorre-Esteves, and D. A. Sinclair. 2002. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J. Biol. Chem. 277:45099-45107. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
