The three-dimensional architecture of the class I ligase ribozyme
- PMID: 14730016
- PMCID: PMC1370529
- DOI: 10.1261/rna.5177504
The three-dimensional architecture of the class I ligase ribozyme
Abstract
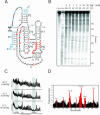
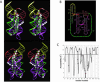
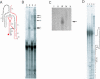
The class I ligase ribozyme catalyzes a Mg(++)-dependent RNA-ligation reaction that is chemically analogous to a single step of RNA polymerization. Indeed, this ribozyme constitutes the catalytic domain of an accurate and general RNA polymerase ribozyme. The ligation reaction is also very rapid in both single- and multiple-turnover contexts and thus is informative for the study of RNA catalysis as well as RNA self-replication. Here we report the initial characterization of the three-dimensional architecture of the ligase. When the ligase folds, several segments become protected from hydroxyl-radical cleavage, indicating that the RNA adopts a compact tertiary structure. Ribozyme folding was largely, though not completely, Mg(++) dependent, with a K(1/2[Mg]) < 1 mM, and was observed over a broad temperature range (20 degrees C -50 degrees C). The hydroxyl-radical mapping, together with comparative sequence analyses and analogy to a region within 23S ribosomal RNA, were used to generate a three-dimensional model of the ribozyme. The predictive value of the model was tested and supported by a photo-cross-linking experiment.
Figures

References
-
- Ban, N., Nissen, P., Hansen, J., Moore, P.B., and Steitz, T.A. 2000. The complete atomic structure of the large ribosomal subunit at 2.4 Å resolution. Science 289: 905–920. - PubMed
-
- Bartel, D.P. 1999. Re-creating an RNA replicase. In The RNA world, 2nd ed. (eds. R.F. Gesteland et al.), pp. 143–162. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
-
- Bergman, N.H., Johnston, W.K., and Bartel, D.P. 2000. Kinetic framework for ligation by an efficient RNA ligase ribozyme. Biochemistry 39: 3115–3123. - PubMed
-
- Cate, J.H., Gooding, A.R., Podell, E., Zhou, K., Golden, B.L., Kundrot, C.E., Cech, T.R., and Doudna, J.A. 1996. Crystal structure of a group I ribozyme domain: Principles of RNA packing. Science 273: 1678–1685. - PubMed
-
- Celander, D.W. and Cech, T.R. 1990. Iron(II)-ethylenediaminetetraacetic acid catalyzed cleavage of RNA and DNA oligonucleotides: Similar reactivity toward single- and double-stranded forms. Biochemistry 29: 1355–1361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
