eIF4A3 is a novel component of the exon junction complex
- PMID: 14730019
- PMCID: PMC1370532
- DOI: 10.1261/rna.5230104
eIF4A3 is a novel component of the exon junction complex
Abstract
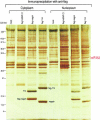
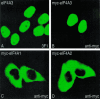
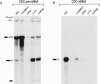
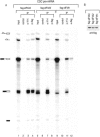
The exon junction complex (EJC) is a protein complex that assembles near exon-exon junctions of mRNAs as a result of splicing. EJC proteins play important roles in postsplicing events including mRNA export, cytoplasmic localization, and nonsense-mediated decay. Recent evidence suggests that mRNA translation is also influenced by the splicing history of the transcript. Here we identify eIF4A3, a DEAD-box RNA helicase and a member of the eIF4A family of translation initiation factors, as a novel component of the EJC. We show that eIF4A3 associates preferentially with nuclear complexes containing the EJC proteins magoh and Y14. Furthermore, eIF4A3, but not the highly related eIF4A1 or eIF4A2, preferentially associates with spliced mRNA. In vitro splicing and mapping experiments demonstrate that eIF4A3 binds mRNAs at the position of the EJC. Using monoclonal antibodies, we show that eIF4A3 is found in the nucleus whereas eIF4A1 and eIF4A2 are found in the cytoplasm. Thus, eIF4A3 likely provides a splicing-dependent influence on the translation of mRNAs.
Figures





References
-
- Bochnig, P., Reuter, R., Bringmann, P., and Lührmann, R. 1987. A monoclonal antibody against 2,2,7-trimethylguanosine that reacts with intact, class U, small nuclear ribonucleoproteins as well as with 7-methylguanosine-capped RNAs. Eur. J. Biochem. 168: 461–467. - PubMed
-
- Dostie, J. and Dreyfuss, G. 2002. Translation is required to remove Y14 from mRNAs in the cytoplasm. Curr. Biol. 12: 1060–1067. - PubMed
-
- Dreyfuss, G., Matunis, M.J., Pinol-Roma, S., and Burd, C.G. 1993. hnRNP proteins and the biogenesis of mRNA. Annu. Rev. Biochem. 62: 289–321. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
