Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein
- PMID: 14732699
- PMCID: PMC327153
- DOI: 10.1073/pnas.0305803101
Increased soluble amyloid-beta peptide and memory deficits in amyloid model mice overexpressing the low-density lipoprotein receptor-related protein
Abstract
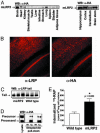
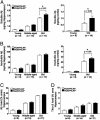
Amyloid-beta peptide (Abeta) is central to the pathogenesis of Alzheimer's disease, and the low-density lipoprotein receptor-related protein (LRP) has been shown to alter Abeta metabolism in vitro. Here, we show that overexpression of a functional LRP minireceptor in the brain of PDAPP mice results in age-dependent increase of soluble brain Abeta, with no changes in Abeta plaque burden. Importantly, soluble brain Abeta was found to be primarily in the form of monomers/dimers and to be highly correlated with deficits in spatial learning and memory. These results provide in vivo evidence that LRP may contribute to memory deficits typical of Alzheimer's disease by modulating the pool of small soluble forms of Abeta.
Figures


References
-
- Hardy, J. & Selkoe, D. J. (2002) Science 297, 353–356. - PubMed
-
- Hardy, J. A. & Higgins, G. A. (1992) Science 256, 184–185. - PubMed
-
- Lendon, C. L., Talbot, C. J., Craddock, N. J., Han, S. W., Wragg, M., Morris, J. C. & Goate, A. M. (1997) Neurosci. Lett. 222, 187–190. - PubMed
-
- Wavrant-DeVrieze, F., Lambert, J. C., Stas, L., Crook, R., Cottel, D., Pasquier, F., Frigard, B., Lambrechts, M., Thiry, E., Amouyel, P., et al. (1999) Hum. Genet. 104, 432–434. - PubMed
-
- Narita, M., Holtzman, D. M., Schwartz, A. L. & Bu, G. (1997) J. Neurochem. 69, 1904–1911. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

