Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis
- PMID: 14734523
- PMCID: PMC2211768
- DOI: 10.1084/jem.20030850
Activation of arterial wall dendritic cells and breakdown of self-tolerance in giant cell arteritis
Abstract
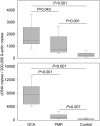
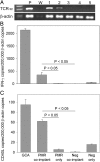
Giant cell arteritis (GCA) is a granulomatous and occlusive vasculitis that causes blindness, stroke, and aortic aneurysm. CD4(+) T cells are selectively activated in the adventitia of affected arteries. In human GCA artery-severe combined immunodeficiency (SCID) mouse chimeras, depletion of CD83(+) dendritic cells (DCs) abrogated vasculitis, suggesting that DCs are critical antigen-presenting cells in GCA. Healthy medium-size arteries possessed an indigenous population of DCs at the adventitia-media border. Adoptive T cell transfer into temporal artery-SCID mouse chimeras demonstrated that DCs in healthy arteries were functionally immature, but gained T cell stimulatory capacity after injection of lipopolysaccharide. In patients with polymyalgia rheumatica (PMR), a subclinical variant of GCA, adventitial DCs were mature and produced the chemokines CCL19 and CCL21, but vasculitic infiltrates were lacking. Human histocompatibility leukocyte antigen class II-matched healthy arteries, PMR arteries, and GCA arteries were coimplanted into SCID mice. Immature DCs in healthy arteries failed to stimulate T cells, but DCs in PMR arteries could attract, retain, and activate T cells that originated from the GCA lesions. We propose that in situ maturation of DCs in the adventitia is an early event in the pathogenesis of GCA. Activation of adventitial DCs initiates and maintains T cell responses in the artery and breaks tissue tolerance in the perivascular space.
Figures





References
-
- Weyand, C.M., and J.J. Goronzy. 2003. Vasculitis of medium- and large-size arteries. N. Engl. J. Med. 349:160–169. - PubMed
-
- Weyand, C.M. 2000. The Dunlop-Dottridge lecture: the pathogenesis of giant cell arteritis. J. Rheumatol. 27:517–522. - PubMed
-
- Hunder, G.G., and R.M. Valente. 2002. Giant cell arteritis: clinical aspects. Inflammatory Diseases of Blood Vessels. G.S. Hoffman and C.M. Weyand, editors. Marcel Dekker, New York. 425–441.
-
- Björnsson, J. 2002. Histopathology of primary vasculitic disorders. Inflammatory Diseases of Blood Vessels. G.S. Hoffman and C.M. Weyand, editors. Marcel Dekker, New York. 255–265.
Publication types
MeSH terms
Substances
Grants and funding
- R01 AR41974/AR/NIAMS NIH HHS/United States
- R01 AR042527/AR/NIAMS NIH HHS/United States
- R01 HL063919/HL/NHLBI NIH HHS/United States
- R01 AI044142/AI/NIAID NIH HHS/United States
- R01 AR041974/AR/NIAMS NIH HHS/United States
- AR07610/AR/NIAMS NIH HHS/United States
- R01 EY11916/EY/NEI NIH HHS/United States
- R01 HL 63919/HL/NHLBI NIH HHS/United States
- R01 AR42527/AR/NIAMS NIH HHS/United States
- R01 CA54464/CA/NCI NIH HHS/United States
- R01 CA81776/CA/NCI NIH HHS/United States
- R01 AI44142/AI/NIAID NIH HHS/United States
- R01 AG15043/AG/NIA NIH HHS/United States
- T32 AR007610/AR/NIAMS NIH HHS/United States
- R01 EY011916/EY/NEI NIH HHS/United States
- R01 AG015043/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

